Subprojects
The subprojects of the CRC1066 consist of the projects of subareas A and B as well as the cross-sectional projects (Q) and the central projects (Z).
In subarea A, very different carriers are provided from the chemical side, taking into account both complex covalently linked polymer architectures and self-assembling structures.
This project combines the use of carbohydrates, both as structural material for the preparation of nanoparticles and nanocapsules and as targeting moieties to achieve selective binding to certain immune cells. By working with polysaccharide based nanocapsules with high loading efficiencies it aims to prepare stimulus responsive carriers, which bind selectively to certain subpopulations of immune cells to release their cargo.
T. Opatz, K. Landfester
Project relevant publications
Krumb M§, Frey ML§, Langhanki J, Forster R, Kowalczyk D, Mailänder V, Landfester K,
Krumb M, Jäger M, Voss A, Immig L, Peters K, Kowalczyk D, Bufe A, Opatz T*, Holst O, Vogel C, Peters M*. Total Synthesis of a Partial Structure from Arabinogalactan and its Application for Allergy-Prevention. (2020) Chem. Eur. J.
Wagener K, Bros M, Krumb M, Langhanki J, Pektor S, Worm M, Schinnerer M, Montermann E, Miederer M, Frey H, Opatz T, Rösch F. Targeting of Immunce Cells with Trimannosylated Liposomes. (2020) Adv. Ther. 1900185.
Tabujew I, Willig M, Leber N, Freidel C, Negwer I, Koynov K, Helm M, Landfester K, Zentel R, Peneva K, Mailänder V. Overcoming the barrier of CD8(+) T cells: Two types of nano-sized carriers for siRNA transport. (2019) Acta Biomater. 100:338-351.
Krumb M, Kammer LM, Forster R, Grundke C, Opatz T*. Visible Light-Induced Cleavage of C–S bonds in Thioacetals and Thioketals with Iodine as a Photocatalyst. (2019) ChemPhotoChem. 4:101-104. (Very Important Paper and Front Cover)
Simon J, Bauer KN, Langhanki J, Opatz T, Mailänder V, Landfester K, Wurm FR. Noncovalent Targeting of Nanocarriers to Immune Cells with Polyphosphoester-Based Surfactants in Human Blood Plasma. (2019) Adv. Sci. 6:1901199.
Kramer S, Langhanki J, Krumb M, Opatz T, Bros M, Zentel R*. HPMA-Based Nanocarriers for Effective Immune System Stimulation. (2019) Macromol. Biosci. 1800481.
Schlegel I, Renz P, Simon J, Lieberwirth I, Pektor S, Bausbacher N, Miederer M, Mailänder V, Munoz-Espi R, Crespy D, Landfester K. Highly Loaded Semipermeable Nanocapsules for Magnetic Resonance Imaging. (2018) Macromol. Biosci. 18(4):e1700387.
Krumb M, Lucas T, Opatz T*. Visible Light Enables Aerobic Iodine Catalyzed Glycosylation. (2019) Eur. J. Org. Chem. 4517-4521. (Front Cover)
Simon J, Christmann S, Mailänder V, Wurm FR, Landfester K. Protein Corona Mediated Stealth Properties of Biocompatible Carbohydrate-based Nanocarriers. (2018) Isr. J. Chem. 58:1363-1372
This project aims to control morphology and function of brush-like peptidic carriers, which are either derived from living polymerization or result from (further processed) natural monodisperse peptides. In addition, peptide based targeting units are modified to allow selective ligation. In combination this project aims at the preparation of systems to study, how morphology (rod or sphere like structures) and/or the ligation of antibodies modify the interaction of the carriers with immune cells.
M. Barz, T. Weil
Project relevant publications
Chen C, Ng DYW, Weil T. Polymer bioconjugates: Modern design concepts toward precision hybrid materials. (2020) Progr. Polym. Sci. 101241.
Alberg I, Kramer S, Schinnerer M, Seidl C, Drude N, Lammers T, Tenzer S, M Barz, R. Zentel. Polymeric Nanoparticles with Neglectable Protein Corona. (2020) Small 16(18):1907574.
Sun Q, Barz M, De Geest BG, Diken M, Kiessling F, Lammers T, Shi Y. Nanomedi-cine and macroscale materials in immuno-oncology. (2019) Chem. Soc. Rev. 48(1):351-381.
Alkilany AM, Weller H, Mews A, Parak WJ, Barz M, Feliu N. Ligand density on nanoparticles: A parameter with critical impact on nanomedicine. (2019) Adv. Drug Deliv. Rev. 143:22-36.
Chen C, Wunderlich K, Mukherji D, Koynov K, Heck AJ, Raabe M, Barz M, Fytas G, Kremer K, Ng DYW, Weil T. Precision anisotropic brush polymers by sequence controlled chemistry. (2019) J. Am. Chem. Soc. 142(3):1332-1340.
Negwer I, Best A, Schinnerer M, Schäfer O, Capeloa L, Wagner M, Schmidt M, Mailänder V, Barz M, Butt HJ, Koynov K. Monitoring drug nanocarriers in human blood by near-infrared fluorescence correlation spectroscopy. (2018) Nat Commun. 9(1):5306.
Morsbach S, Gonella G, Mailander V, Wegner S, Weidner T, Berger R, Koynov K, Vollmer D, Encinas N, Kuan SL, Kremer K, Weil T, Bonn M, Butt HJ, Landfester K. Engineering Proteins at Interfaces: From Complementary Characterization to Material Surfaces with Designed Functions. (2018) Angew. Chem. Internat. Ed. 57(39):12626-12648.
Agrawalla BK, Wang T, Riegger A, Domogalla MP, Steinbrink K, Dörfler T, Chen X, Boldt F, Michaelis J, Kuan SL, Weil T. Chemoselective Dual Labeling of Native and Recombinant Proteins. (2018) Bioconjug. Chem. 29(1):29-34.
Kuan SL, Wang T, Weil T. Site-Selective Disulfide Modification of Proteins – Expanding Diversity beyond the Proteome. (2016) Chem. Europ. J.
Hörtz C, Birke A, Kaps L, Decker S, Wächtersbach E, Fischer K, Schuppan D, Barz M, Schmidt M. Cylindrical Brush Polymers with Polypept(o)ide Side Chains: A Novel Biocompatible Carrier for Biomedical Applications. (2015) Macromolecules 48(7):2074-2086.
Within this project the processing and loading (formulation) of polymersomes and liposomes as car-riers for peptides, RNA and/or low molar mass pharmacons will be studied. It is thereby the speciality of these polymersomes that they can be coated with hyperbranched PEG and decorated with targeting structures. To modify the release as recondition for biomedical activity the stability of the basic polymersomal structure will be modified to allow stabilization (e.g. crosslinking) or destabiliza-tion by pH dependent degradation.
H. Frey, M. Helm, R. Zentel
Project relevant publications
Wagener K, Bros M, Krumb M, Langhanki J, Pektor S, Worm M, Schinnerer M, Montermann E, Miederer M, Frey H, Opatz T, Rösch F. Targeting of Immune Cells with Trimannosylated Liposomes. (2020) Adv. Therap. 3:1900185.
Weber C, Voigt M, Simon J, Danner AK, Frey H, Mailänder V, Helm M, Morsbach S, Landfester K. Functionalization of Liposomes with Hydrophilic Polymers Results in Macrophage Uptake Independent of the Protein Corona. (2019) Biomacromolecules. 20(8):2989-2999.
Danner AK, Schöttler S, Alexandrino E, Hammer S, Landfester K, Mailänder V, Morsbach S, Frey H, Wurm FR. Phosphonylation Controls the Protein Corona of Multifunctional Polyglycerol Modified Nanocarriers. (2019) Macromol Biosci. 19(5):1800468.
Pannwitt S, Slama K, Depoix F, Helm M, Schneider D. Against expectations: Unassisted RNA adsorption onto negatively charged lipid bilayers. (2019) Langmuir. 35(45):14704-14711.
Negwer I, Best A, Schinnerer M, Schäfer O, Capeloa L, Wagner M, Schmidt M, Mailänder V, Helm M, Barz M, Butt H-J, Koynov K. Monitoring drug nanocarriers in human blood by near-infrared fluorescence correlation spectroscopy. (2018) Nature Communications. 9(1):5306.
Wagener K, Worm M, Pektor S, Schinnerer M, Thiermann R, Miederer M, Frey H, Rösch F. Comparison of Linear and Hyperbranched Polyether Lipids for Liposome Shielding by 18F-Radiolabeling and Positron Emission Tomography. (2018) Biomacromolecules. 19:2506.
Hellmuth I, Freund I, Schlöder J, Seidu-Larry S, Thüring K, Slama K, Langhanki J, Kaloyanova S, Eigenbrod T, Krumb M, Röhm S, Peneva K, Opatz T, Jonuleit H, Dalpke AH, Helm M. Bioconjugation of Small Molecules to RNA Impedes Its Recognition by Toll-Like Receptor. (2017) Fronti. Immunol. 8:312.
Bartneck M, Schlößer C, Barz M, Zentel R, Trautwein C, Lammers T, Tacke F. Immunomodulatory Therapy of Inflammatory Liver Disease Using Selectin-Binding Glycopolymers. (2017) ACS Nano. 11:9689-9700.
Kramer S, Kim KO, Zentel R. Size Tunable Core Crosslinked Micelles from HPMA Based Amphiphilic Block Copolymers. (2017) Macromol. Chem. Phys. 218:1700113.
Scherer M, Kappel C, Mohr N, Fischer K, Heller P, Forst R, Depoix F, Bros M, Zentel R. Functionalization of Active Ester-Based Polymersomes for Enhanced Cell Uptake and Stimuli-Responsive Cargo Release. (2016) Biomacromolecules. 17:3305-3317.
The cross-sectional projects (Q) form the natural bridge between the A and B projects. They are of central importance for the majority of the subprojects in the A and B areas and are characterized by a very high CRC-specific research content.
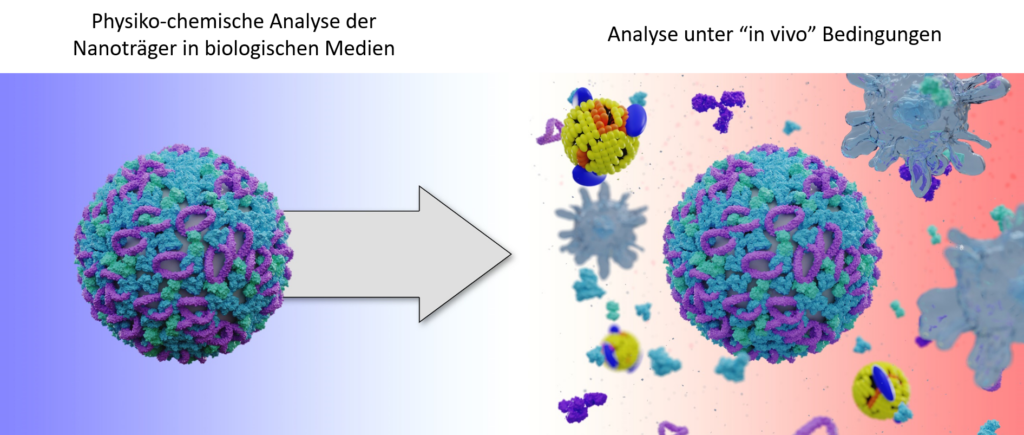
The goal of TP Q1 is to analyze interactions between nanocarriers and blood components. Molecular determinants will be analyzed that lead to enhanced in vivo uptake and improved efficacy of the intended immunomodulation induced by polymer- and lipid- based NP developed within the CRC1066, with a focus on the protein corona, protein-lipid interactions, and the role of lipids in general. This is done by physico-chemical characterization methods, molecular simulations, and data-driven modelling. The protein corona is analyzed by dynamic light scattering, mass spectrometry, microcalorimetry, and a recently established AF-FFF-based separation technique.
K. Landfester, M. Maskos, F. Schmid
Project relevant publications
Alberg I, Kramer S, Schinnerer M, Qizhi H, Seidl C, Leps C, Drude N, Möckel D, Rijcken C, Lammers T, Diken M, Maskos M, Morsbach S, Landfester K, Tenzer S, Barz M, Zentel R. Polymeric nanoparticles with neglectable protein corona. (2020) Small. 18:1907574.
Prozeller D, Pereira J, Simon J, Mailänder V, Morsbach S, Landfester K. Prevention of Dominant IgG Adsorption on Nanocarriers in IgG-Enriched Blood Plasma by Clusterin Precoating. (2020) Adv. Sci. 6:1802199.
Steen EJL, Jorgensen JT, Johann K, Norregaard K, Sohr B, Svatunek D, Birke A, Shalgunov V, Edem PE, Rossin R, Seidl C, Schmid F, Robillard MS, Kristensen JL, Mikula H, Barz M, Kjaer A, Herth MM. Trans-Cyclooctene-Functionalized PeptoBrushes with Improved Reaction Kinetics of the Tetrazine Ligation for Pretargeted Nuclear Imaging. (2020) ACS Nano. 14:568-584.
Weber C, Morsbach S, Landfester K. Possibilities and Limitations of Different Separation Techniques for the Analysis of the Protein Corona. (2019) Angew. Chem. Int. Ed. 58:12787.
Mantha S, Qi SH, Barz M, Schmid F. How ill-defined constituents produce well-defined nanoparticles: Effect of polymer dispersity on the uniformity of copolymeric micelles. (2019) Phys. Rev. Mat. 3:026002.
Simon J, Müller J, Ghazaryan A, Morsbach S, Mailänder V, Landfester K. Protein denaturation caused by heat inactivation detrimentally affects biomolecular corona for-mation and cellular uptake. (2018) Nanoscale. 10:21096-21105.
Settanni G, Schäfer T, Muhl C, Barz M, Schmid F. Poly-sarcosine and Poly(Ethylene-Glycol) Interactions with Proteins Investigated Using Molecular Dynamics Simulations. (2018) Comp. Struct. Biot. J. 16:543-550.
Thiermann R, Bleul R, Maskos M. Kinetic control of block copolymer self-assembly in a micromixing device – mechanistical insight into vesicle formation process. (2017) Macromol. Chem. Phys. 218(2):1600347.
Settanni G, Zhou JJ, Suo TC, Schottler S, Landfester K, Schmid F, Mailänder V. Protein corona composition of poly(ethylene glycol)- and poly(phosphoester)-coated nanoparticles correlates strongly with the amino acid composition of the protein surface. (2017) Nanoscale. 9:2138-2144.
Heller P, Zhou JJ, Weber B, Hobernik D, Bros M, Schmid F, Barz M. The Influence of Block lonomer Microstructure on Polyplex Properties: Can Simulations Help to Understand Differences in Transfection Efficiency? (2017) Small. 13:1603694.
Observation of interaction forces by investigation of the influence of eluent additives on the retention behavior of aqueous nanoparticle dispersions in asymmetrical flow field-flow fractionation Conrad Nickel a , ∗, Christian Scherer a , Sergey Noskov a , Christoph Bantz b , Martin Berger a , Wolfgang Schupp c , Michael Maskos a , b; Journal of Chromatography A
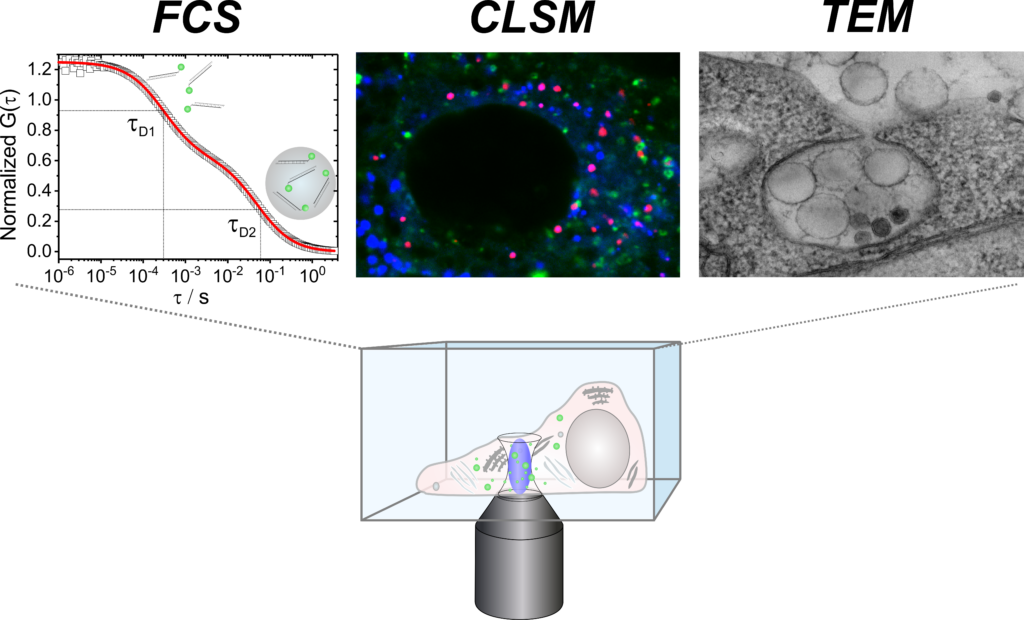
Q2 will focus on the characterization of the nanocarriers (NC) and their interactions with biological matter. Ex vivo fluorescence correlation spectroscopy will study the NC kinetics in the blood of living animals with respect to possible aggregation, degradation or premature release of cargo. Furthermore, the structure of the NCs protein corona and its influence on endocytosis processes will be reconstructed by 3D cryo electron microscopy. The type and effectiveness of intracellular drug release from NCs, as well as the effective-ness of major histocompatibility complex (MHC) loading and stimulation of T cells, will be analyzed by proteomics of intracellular compartments and by transcriptome analysis using RNASeq. ex-vivo-Fluorezenz-Korrelationsspektroskopie wird die NC-Kinetik im Blut lebender Tiere bezüglich Aggregation, Degradation oder vorzeitiger Wirkstoff-Freisetzung untersuchen. Die Struktur der NC-Proteinkorona und deren Einfluss auf die Endozytose wird mittels 3D Cryo-Elektronenmikroskopie untersucht. Weiterhin sollen Art und Effektivität der intrazellulären Wirkstofffreisetzung wie die Beladung des Major Histocompatibility Complexes (MHC) sowie die Stimulation von T-Zellen durch Proteomics der intrazellulären Kompartimente und durch Transkriptom-Analysen mittels RNASeq analysiert werden.
K. Koynov, I. Lieberwirth, V. Mailänder
Project relevant publications seit 2017:
Jiang S, Prozeller D, Pereira J, Simon J, Han S, Wirsching S, Fichter M, Mottola M, Lieberwirth I, Morsbach S, Mailander V, Gehring S, Crespy D, Landfester K. Controlling protein interactions in blood for effective liver immunosuppressive therapy by silica nanocapsules. (2020) Nanoscale. 12:2626-2637.
Gai M, Simon J, Lieberwirth I, Mailander V, Morsbach S, Landfester K. A bio-orthogonal functionalization strategy for site-specific coupling of antibodies on vesicle surfaces after self-assembly. (2020) Polymer Chemistry. 11:527-540.
Holm R, Schwiertz D, Weber B, Schultze J, Kuhn J, Koynov K, Lächelt U, Barz M. Multifunctional Cationic PeptoStars as siRNA Carrier: Influence of Architecture and Histidine Modification on Knockdown Potential. (2020) Macromolecular Bioscience. 20:1900152.
Tabujew I, Willig M, Leber N, Freidel C, Negwer I, Koynov K, Helm M, Landfester K, Zentel R, Peneva K, Mailander V. Overcoming the barrier of CD8(+) T cells: Two types of nano-sized carriers for siRNA transport. (2019) Acta Biomaterialia. 100:338-351.
Kramer S, Svatunek D, Alberg I, Grafen B, Schmitt S, Braun L, van Onzen A, Rossin R, Koynov K, Mikula H, Zentel R. HPMA-Based Nanoparticles for Fast, Bioorthogonal iEDDA Ligation. (2019) Biomacromolecules. 20:3786-3797.
Guindani C, Frey ML, Simon J, Koynov K, Schultze J, Ferreira SRS, Araujo PHH, de Oliveira D, Wurm FR, Mailander V, Landfester K. Covalently Binding of Bovine Serum Albumin to Unsaturated Poly(Globalide-Co-epsilon-Caprolactone) Nanoparticles by Thiol-Ene Reactions. (2019) Macromolecular Bioscience. 19:1900145.
Reinholz J, Diesler C, Schöttler S, Kokkinopoulou M, Ritz S, Landfester K, Mailander V. Protein machineries defining pathways of nanocarrier exocytosis and transcytosis. (2018) Acta Biomaterialia. 71:432-443.
Negwer I, Best A, Schinnerer M, Schafer O, Capeloa L, Wagner M, Schmidt M, Mailander V, Helm M, Barz M, Butt HJ, Koynov K. Monitoring drug nanocarriers in human blood by near-infrared fluorescence correlation spectroscopy. (2018) Nature Communications. 9:5306.
Morsbach S, Gonella G, Mailander V, Wegner S, Wu S, Weidner T, Berger R, Koynov K, Vollmer D, Encinas N, Kuan S, Bereau T, Kremer K, Weil T, Bonn M, Butt HJ, Landfester K. Engineering Proteins at Interfaces: From Complementary Characterization to Material Surfaces with Designed Functions. (2018) Angewandte Chemie-International Edition. 57:12626-12648.
Nuhn L, Van Herck S, Best A, Deswarte K, Kokkinopoulou M, Lieberwirth I, Koynov K, Lambrecht BN, De Geest BG. FRET Monitoring of Intracellular Ketal Hydrolysis in Synthetic Nanoparticles. (2018) Angewandte Chemie-International Edition. 57:10760-10764.
oynov
This project aims at in vivo imaging of the biodistribution of nanodimensional carriers on different length scales (from organ to cellular assembly) by PET- and optical methods. It is thereby the inten-tion to validate fluorescence imaging against the quantitative PET method. In addition, methods to visualize the activation of the immune system (immuno-imaging) as result of the immunotherapy will be developed.
T. Lammers, M. Miederer, F. Rösch
Project relevant publications seit 2017:
Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D, Kuhn AN, Omokoko T, Kranz LM, Diken M, Kreiter S, Haas H, Attig S, Rae R, Cuk K, Kemmer-Bruck A, Breitkreuz A, Tolliver C, Caspar J, Quinkhardt J, Hebich L, Stein M, Hohberger A, Vogler I, Liebig I, Renken S, Sikorski J, Leierer M, Muller V, Mitzel-Rink H, Miederer M, Huber C, Grabbe S, Utikal J, Pinter A, Kaufmann R, Hassel JC, Loquai C, Tureci O. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. (2020) Nature. 585:107-112.
Pektor S, Schlöder J, Klasen B, Bausbacher N, Wagner DC, Schreckenberger M, Grabbe S, Jonuleit H, Miederer M. Using immuno-PET imaging to monitor kinetics of T cell-mediated inflammation and treatment efficiency in a humanized mouse model for GvHD. (2020) Eur J Nucl Med Mol I. 47:1314-1325.
Wagener K, Bros M, Krumb M, Langhanki J, Pektor S, Worm M, Schinnerer M, Montermann E, Miederer M, Frey H, Opatz T, Rosch F. Targeting of Immune Cells with Trimannosylated Lip-osomes. (2020) Adv Ther-Germany. 3:1900185.
May JN, Golombek SK, Baues M, Dasgupta A, Drude N, Rix A, Rommel D, von Stillfried S, Appold L, Pola R, Pechar M, van Bloois L, Storm G, Kuehne AJC, Gremse F, Theek B, Kiessling F, Lammers T. Multimodal and multiscale optical imaging of nanomedicine delivery across the blood-brain barrier upon sonopermeation. (2020) Theranostics. 10:1948-1959.
Alberg I, Kramer S, Schinnerer M, Hu QZ, Seidl C, Leps C, Drude N, Möckel D, Rijcken C, Lammers T, Diken M, Maskos M, Morsbach S, Landfester K, Tenzer S, Barz M, Zentel R. Polymeric Nanoparticles with Neglectable Protein Corona. (2020) Small. 16.
Stergiou N, Nagel J, Pektor S, Heimes AS, Jakel J, Brenner W, Schmidt M, Miederer M, Kunz H, Roesch F, Schmitt E. Evaluation of a novel monoclonal antibody against tumor-associated MUC1 for diagnosis and prognosis of breast cancer. (2019) Int J Med Sci. 16(9):1188-1198.
Theek B, Baues M, Gremse F, Pola R, Pechar M, Negwer I, Koynov K, Weber B, Barz M, Dechent W, Storm G, Kiessling F, Lammers T. Histidine-rich glycoprotein-induced vascular normalization improves EPR-mediated drug targeting to and into tumors. (2018) J Control Release. 282:25-34.
Eppard E, de la Fuente A, Mohr N, Allmeroth M, Zentel R, Miederer M, Pektor S, Rosch F. Labeling of DOTA-conjugated HPMA-based polymers with trivalent metallic radionuclides for molecular imaging. (2018) EJNMMI Research. 8:16.
Wagener K, Worm M, Pektor S, Schinnerer M, Thiermann R, Miederer M, Frey H, Rosch F. Comparison of Linear and Hyperbranched Polyether Lipids for Liposome Shielding by F-18-Radiolabeling and Positron Emission Tomography. (2018) Biomacromolecules. 19:2506-2516.
Pektor S, Hilscher L, Walzer KC, Miederer I, Bausbacher N, Loquai C, Schreckenberger M, Sahin U, Diken M, Miederer M. In vivo imaging of the immune response upon systemic RNA cancer vaccination by FDG-PET. (2018) Ejnmmi Res. 8.
In TP Q4, human-relevant animal models and in vitro systems as alternatives to animal models, which are of use for the entire SFB, will be further developed and characterized by investigating the genetic variability and neoantigen expression in spontaneously developing murine melanomas and by establishing methods for standardized procedures of immunotherapy experiments in spontaneous tumor models. Furthermore, animal model alternatives, such as tumor organoids will further be improved to analyse changes in the tumor microenvironment and to investigate the effects of NP on the tumor-specific immune response.
M. Diken, H. Schild
Project relevant publications
Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D, Kuhn AN, Omokoko T, Kranz LM, Diken M, Kreiter S, Haas H, Attig S, Rae R, Cuk K, Kemmer-Brück A, Breitkreuz A, Tolliver C, Caspar J, Quinkhardt J, Hebich L, Stein M, Hohberger A, Vogler I, Liebig I, Renken S, Sikorski J, Leierer M, Müller V, Mitzel-Rink H, Miederer M, Huber C, Grabbe S, Utikal J, Pinter A, Kaufmann R, Hassel JC, Loquai C, Türeci Ö. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. (2020) Nature. 585(7823):107-112.
Scherger M, Bolli E, Antunes ARP, Arnouk S, Stickdorn J, Van Driessche A, Grabbe S, Schild H, De Geest BG, Van Ginderachter JA, Nuhn L. Transient Multivalent Nanobody Targeting to CD206-expressing Cells via pH-Degradable Nanogels. (2020) Cells. 9(10), 2222.
Alberg I, Kramer S, Schinnerer M, Hu Q, Seidl C, Leps C, Drude N, Möckel D, Rijcken C, Lammers T, Diken M, Maskos M, Morsbach S, Landfester K, Tenzer S, Barz M, Zentel R. Polymeric Nanoparticles with Neglectable Protein Corona. (2020) Small. 16(18):e1907574.
Reinhard K, Rengstl B, Oehm P, Michel K, Billmeier A, Hayduk N, Klein O, Kuna K, Ouchan Y, Wöll S, Christ E, Weber D, Suchan M, Bukur T, Birtel M, Jahndel V, Mroz K, Hobohm K, Kranz L, Diken M, Kühlcke K, Türeci Ö, Sahin U. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. (2020) Science. 367(6476):446-453.
Grunwitz C, Salomon N, Vascotto F, Selmi A, Bukur T, Diken M, Kreiter S, Türeci Ö, Sahin U. HPV16 RNA-LPX vaccine mediates complete regression of aggressively growing HPV-positive mouse tumors and establishes protective T cell memory. (2019) Oncoimmunology. 8(9):e1629259.
Bialojan A, Sohl J, Rausch J, Aranda Lopez P, Denny M, Langguth P, Hartmann AK, Yagita H, Probst HC, Schild H, Radsak MP. Transcutaneous immunization with CD40 ligation boosts cytotoxic T lymphocyte mediated anti-tumor immunity independent of CD4 helper T cells in mice. (2019) Eur. J. Immunol. 49(11):2083-94.
Shen L, Tenzer S, Storck W, Hobernik D, Raker VK, Fischer K, Decker S, Dzionek A, Krauthäuser S, Diken M, Nikolaev A, Maxeiner J, Schuster P, Kappel C, Verschoor A, Schild H*, Grabbe S*, Bros M*. Protein corona-mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses. (2018) J Allergy Clin Immunol. 142(5):1558-1570. *equally contributed to last authorship
Vormehr M, Reinhard K, Blatnik R, Josef K, Beck JD, Salomon N, Suchan M, Selmi A, Vascotto F, Zerweck J, Wenschuh H, Diken M, Kreiter S, Türeci Ö, Riemer AB, Sahin U. A non-functional neoepitope specific CD8+ T-cell response induced by tumor derived antigen exposure in vivo. (2018) Oncoimmunology. 8(3):1553478.
Shen L, Krauthäuser S, Fischer K, Hobernik D, Abassi Y, Dzionek A, Nikolaev A, Voltz N, Diken M, Krummen M, Montermann E, Tubbe I, Lorenz S, Strand D, Schild H, Grabbe S, Bros M. Vaccination with trifunctional nanoparticles that address CD8+ dendritic cells inhibits growth of established melanoma. (2016) Nanomedicine (Lond). 11(20):2647-2662.
Kranz LM*, Diken M*, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, Vormehr M, Hüsemann Y, Selmi A, Kuhn AN, Buck J, Derhovanessian E, Rae R, Attig S, Diekmann J, Jabulowsky RA, Heesch S, Hassel J, Langguth P, Grabbe S, Huber C, Türeci Ö, Sahin U. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. (2016) Nature. 534(7607):396-401. *equally contributed to first authorship.
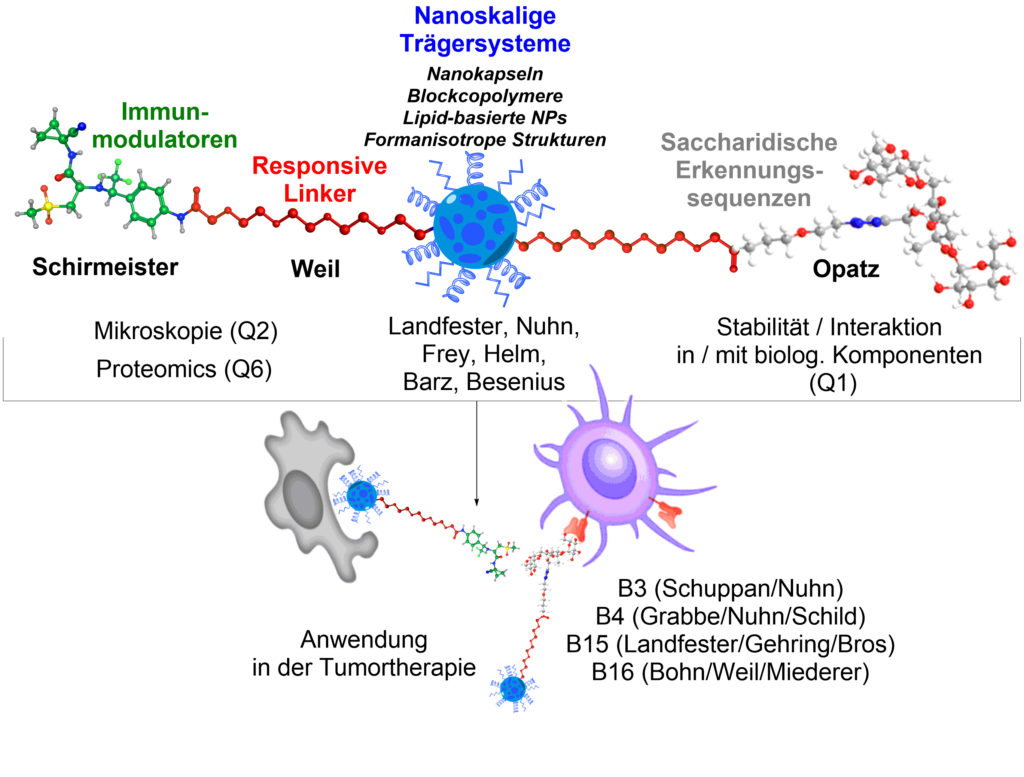
The new project Q5 will further evolve the existing nanocarrier systems within the CRC through new chemical ligation methods and selective targeting structures. For an effective targeting of immune cells, e.g. saccharidic recognition sequences (oligosaccharides) will be attached to the nanocarriers through responsive linker systems. As a practical application of newly developed targeting structures and their conjugation chemistry, inhibitors of the cysteine-cathepsin S, an important regulator of antigen presentation, will be conjugated to nanoparticles or used as a non-covalent payload. The resulting constructs will be evaluated as potential tumor therapeutics.
Opatz, T. Schirmeister, T. Weil
Project relevant publications
Krumb M, Jäger M, Voss A, Immig L, Peters K, Kowalczyk D, Bufe A, Opatz T, Holst O, Vogel C, Peters M. Total Synthesis of a Partial Structure from Arabinogalactan and its Application for Allergy-Prevention. (2020) Chem. Eur.
Wagener K, Bros M, Krumb M, Langhanki J, Pektor S, Worm M, Schinnerer M, Montermann E, Miederer M, Frey H, Opatz T, Rösch F. Targeting of Immune Cells with Trimannosylated Liposomes. (2020) Adv. Therap. 1900185.
Klein P, Barthels F, Johe P, Wagner A, Tenzer S, Distler U, Le TA, Schmid P, Engel V, Engels B, Hellmich UA, Opatz* T, Schirmeister* T. Naphthoquinones as covalent reversible inhibitors of cysteine proteases – Studies on inhibition mechanisms and kinetics. (2020) Molecules. 25:2064.
Klein P, Johé P, Wagner A, Jung S, Engels B, Kühlborn J, Tenzer S, Distler U, Waigel W, Hellmich UA, Opatz* T, Schirmeister* T. New Cysteine Protease Inhibitors: Electrophilic (Het)arenes and unexpected Prodrug Identification. (2020) Molecules. 25:1451.
Fuchs N, Meta M, Schuppan* D, Nuhn* L, Schirmeister* T. Novel opportunities for cathepsin S inhibitors in cancer immunotherapy by nanocarrier-mediated delivery. (2020) Cells. 9:2021.
Xu L, Raabe M, Zegota MM, Nogueira JCF, Chudasama V, Kuan SL, Weil* T. Site-selective protein modification via disulfide rebridging for fast tetrazine/trans-cyclooctene bioconjugation. (2020) Org. Biomol. Chem. 18(6):1140-1147.
Hebel M, Riegger A, Zegota MM, Kizilsavas G, Gačanin J, Pieszka M, Lueckerath T, Coelho JAS, Wagner M, Gois PMP, Ng DYW, Weil* T. Sequence Programming with Dynamic Boronic Acid/Catechol Binary Codes. (2019) J. Am. Chem. Soc. 141(36):14026-14031.
Kramer S, Langhanki J, Krumb M, Opatz T, Bros M, Zentel R. HPMA-Based Nanocarriers for Effective Immune System Stimulation. (2019) Macromol. Biosci.1800481.
Agrawalla BK, Wang T, Riegger A, Domogalla MP, Steinbrink K, Dörfler T, Chen X, Boldt F, Lamla M, Michaelis J, Kuan SL, Weil T. Chemoselective Dual Labelling of Native and Recombinant Proteins. (2018) Bioconj. Chem. 29(1):29-34.
Schirmeister* T, Kesselring J, Jung S, Schneider T, Weickert A, Becker J, Lee W, Bamberger D, Wich P, Distler U, Tenzer S, Johé P, Hellmich UA, Engels* B. Quantum chemical-based protocol for the rational design of covalent inhibitors. (2016) J. Am. Chem. Soc. 138:8332-8335.
The work of TP Q6 will focus on the analysis of protein corona and the biodistribution of nanomaterials after systemic application. Extending the work of previous TP B11 and TP A7, the composition and relevance of the protein corona for blood circulation and the targeting of NP will be analyzed. In particular, the importance of different antibody formats (whole antibody, Fab fragments, scFv, nanobodies) and their suitability for targeting efficiency will be further explored. Furthermore, the effects of different surface modifications (chemical variation of polyether architectures and functionality) on protein corona and in vivo targeting by surface functionalization (Fab antibody fragments, nanobodies) will be investigated.
V. Mailänder, S. Tenzer, F. Frey
Project relevant publications
Wagner J, Li LJ, Simon J, Krutzke L, Landfester K, Mailänder V, Müllen K, Ng DYW, Wu YZ, Weil T. Amphiphilic Polyphenylene Dendron Conjugates for Surface Remodeling of Adenovirus 5. (2020) Angewandte Chemie Int. Ed. 59(14):5712-5720.
Keth J, Johann T, Frey H. Hydroxamic Acid – An Underrated Moiety? Marrying Bioinorganic Chemistry and Polymer Science. (2020) Biomacromolecules. 21(7):2546-2556.
Weber C, Voigt M, Simon J, Danner AK, Frey H, Mailänder V, Helm M, Morsbach S, Landfester K. Functionalization of Liposomes with Hydrophilic Polymers Results in Macrophage Uptake Independent of the Protein Corona. (2019) Biomacromolecules. 20(8):2989-2999.
Alberg I, Kramer S, Schinnerer M, Hu Q, Seidl C, Leps C, Drude N, Möckel D, Rijcken C, Lammers T, Diken M, Maskos M, Morsbach S, Landfester K, Tenzer S*, Barz M*, Zentel R*. Polymeric Nanoparticles with Neglectable Protein Corona. (2020) Small. 16(18):e1907574.
Distler U, Łącki MK, Schumann S, Wanninger M, Tenzer S. Enhancing Sensitivity of Microflow-Based Bottom-Up Proteomics through Postcolumn Solvent Addition. (2019) Anal Chem. 91(12):7510-7515.
Prozeller D, Pereira J, Simon J, Mailänder V, Morsbach S, Landfester K. Prevention of Dominant IgG Adsorption on Nanocarriers in IgG-Enriched Blood Plasma by Clusterin Precoating. (2019) Adv Sci. 6(10):1802199.
Shen L, Tenzer S, Storck W, Hobernik D, Raker VK, Fischer K, Decker S, Dzionek A, Krauthäuser S, Diken M, Nikolaev A, Maxeiner J, Schuster P, Kappel C, Verschoor A, Schild H, Grabbe S, Bros M. (2018) Protein corona-mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses. (2018) J Allergy Clin Immunol. 142(5):1558-1570.
Tonigold M, Simon J, Estupinan D, Kokkinopoulou M, Reinholz J, Kintzel U, Kaltbeitzel A, Renz P, Domogalla MP, Steinbrink K, Lieberwirth I, Crespy D, Landfester K, Mailänder V. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. (2018) Nature Nanotechnology. 13(9):862-869.
Herzberger J, Niederer K, Pohlit H, Seiwert J, Worm M, Wurm FR, Frey H. Polymerization of Ethylene Oxide, Propylene Oxide and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures and Bioconjugation. (2016) Chem. Rev. 116:2170-2243.
Fritz T, Voigt M, Worm M, Negwer I, Müller SS, Kettenbach K, Ross TL, Rösch F, Koynov K, Frey H, Helm M. Orthogonal click conjugation to the liposomal surface reveals stability of lipid anchorage as crucial for targeting. (2016) Chem. Eur J. 33:11578-11582.
Subarea B groups together the projects that are primarily concerned with the application of nanoparticles and polymers for tumor immunotherapy.
M2-type cancer promoting macrophages in the liver cancer microenvironment will be repolarized to cancer suppressing M1-type macrophages using block copolymer-based cationic nanogels. The nanoparticles will be loaded with small molecular drugs, siRNA or antisense oligonucleotides. Targeted delivery and thus a more effective repolarization of M2-macrophages will be further improved by derivatisation of the nanoparticles with CD206-binding peptides or nanobodies. For in vivo efficacy testing we have established 3 syngenic mouse models of hepatocellular carcinoma. in vivo Effektivitätstestungen wurden 3 syngene Mausmodelle des hepatozellulären Karzinoms etabliert.
D. Schuppan, L. Nuhn
Project relevant publications
Bixenmann L, Stickdorn J, Nuhn L*. Amphiphilic poly(esteracetal)s as dual pH- and enzyme-responsive micellar immunodrug delivery systems. (2020) Polym Chem. 11:2441-2456.
Fuchs N, Meta M, Schuppan D*, Nuhn L*, Schirmeister T*. Novel Opportunities for Cathepsin S Inhibitors in Cancer Immmunotherapy by Nanocarrier-mediated Delivery. (2020) Cells. 9(9):2021.
Kaps L, Leber N, Klefenz A, Choteschovsky N, Zentel R, Nuhn L*, Schuppan D*. In Vivo siRNA Delivery to Immunosuppressive Liver Macrophages by α-Mannosyl-Functionalized Cationic Nanohydrogel Particles. (2020) Cells. 9(8):1905.
Schupp J, Krebs FK, Zimmer N, Trzeciak E, Schuppan D*, Tuettenberg A*. Targeting myeloid cells in the tumor sustaining microenvironment. (2019) Cell Immunol. 343(9):103713.
Leber N, Kaps L, Yang A, Aslam M, Giardino M, Rosigkeit S, Mostafa A, Nuhn L*, Schuppan D*, Zentel R*. α-Mannosyl-Functionalized Cationic Nanohydrogel Particles for Targeted Gene Knockdown in Immunosuppressive Macrophages. (2019) Macromol Biosci. 19(7):1900162.
Foerster F, Hess M, Gerhold-Ay A, Marquardt JU, Becker D, Galle PR, Schuppan D, Binder H, Bockamp E. The immune contexture of hepatocellular carcinoma predicts clinical outcome. (2018) Sci Rep. 29(8):5351.
Weng SY, Wang X, Vijayan S, Tang Y, Kim YO, Padberg K, Regen T, Molokanova O, Chen T, Bopp T, Schild H, Brombacher F, Crosby JR, McCaleb ML, Waisman A, Bockamp E, Schuppan D. IL-4 Receptor Alpha Signaling through Macrophages Differentially Regulates Liver Fibrosis Progression and Reversal. (2018) EBioMedicine. 29:92-103.
Nuhn L*, Bolli E, Massa S, Vandenberghe I, Movahedi K, Devreese B, Van Ginderachter JA*, De Geest BG*. Targeting Protumoral Tumor-associated Macrophages with Nanobody-functionalized Nanogels through SPAAC Ligation. (2018) Bioconj Chem. 29(7)2394-2405.
Leber N, Kaps L, Aslam M, Schupp J, Brose A, Schäffel D, Fischer K, Diken M, Strand D, Koynov K, Tuettenberg A, Nuhn L*, Zentel R*, Schuppan D*. SiRNA-Mediated In Vivo Gene Knockdown by Acid-Degradable Cationic Nanohydrogel Particles. (2017) J Controlled Release. 248:10-23.
Leber N, Nuhn L, Zentel R. Cationic Nanohydrogel Particles For Therapeutic Oligonucleotide Delivery. (2017) Macromol Biosci. 17(10):1700092.
Multifunctional nanoparticles equipped with tumor antigens and immunostimulatory TLR-agonists, as well as targeting units for addressing antigen-presenting cells (APC), can trigger an effective immune response against established murine tumors after systemic application. For this purpose, this subproject will establish biodegradable, poly(carbonate-)-based nanogels and characterize their immunotherapeutic efficacy in injected, metastatic and inducible melanoma models. Beyond the relevance of different APC populations and immune effector cells, the role of B-cells for tumor vaccine responses will be deciphered in detail.
S. Grabbe, L. Nuhn, H. Schild
Project relevant publications
Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D, Kuhn AN, Omokoko T, Kranz LM, Diken M, Kreiter S, Haas H, Attig S, Rae R, Cuk K, Kemmer-Brück A, Breitkreuz A, Tolliver C, Caspar J, Quinkhardt J, Hebich L, Stein M, Hohberger A, Vogler I, Liebig I, Renken S, Sikorski J, Leierer M, Müller V, Mitzel-Rink H, Miederer M, Huber C, Grabbe S, Utikal J, Pinter A, Kaufmann R, Hassel JC, Loquai C, Türeci Ö. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. (2020) Nature. 585(7823):107-112.
Schaupp L, Muth S, Rogell L, Kofoed-Branzk M, Melchior F, Lienenklaus S, Ganal-Vonarburg SC, Klein M, Guendel F, Hain T, Schütze K, Grundmann U, Schmitt V, Dorsch M, Spanier J, Larsen PK, Schwanz T, Jäckel S, Reinhardt C, Bopp T, Danckwardt S, Mahnke K, Heinz GA, Mashreghi MF, Durek P, Kalinke U, Kretz O, Huber TB, Weiss S, Wilhelm C, Macpherson AJ, Schild H*, Diefenbach A*, Probst HC*. Microbiota-induced tonic type I interferons instruct a transcriptional, epigenetic and metabolic program that defines the basal state of conventional dendritic cells. (2020) Cell. 181:1-17.
Kockelmann J, Stickdorn J, Kasmi S, De Vrieze J, Pieszka M, Ng, DYW, David SA, De Geest BG, Nuhn L. Control over immune stimulation by pH-degradable poly(norbornene) nanogels. (2020) Biomacromolecules. 21(6):2246-2257.
Scherger M, Bolli E, Antunes ARP, Arnouk S, Stickdorn J, Van Driessche A, Grabbe S, Schild H, De Geest BG, Van Ginderachter JA*, Nuhn L*. Transient multivalent nanobody targeting to CD206-expressing cells via pH-degradable nanogels. (2020) Cells. 9:2222.
Lybaert L, Vermaelen K, De Geest BG, Nuhn L. Immunoengineering through cancer vaccines – a personalized and multi-step vaccine approach towards precise cancer immunity. (2018) J Control Release. 289:125-145.
Paßlick D, Piradashvili K, Bamberger D, Li M, Jiang S, Strand D, R Wich P, Landfester K, Bros M, Grabbe S*, Mailänder V*. Delivering all in one: Antigen-nanocapsule loaded with dual adjuvant yields superadditive effects by DC-directed T cell stimulation. (2018) J Control Release. 289:23-34.
Bros M, Nuhn L, Simon J, Moll L, Mailänder V, Landfester K, Grabbe S. The protein corona as a con-founding variable of nanoparticle-mediated targeted vaccine delivery. (2018) Front Immunol. 9:1760.
Shen L, Tenzer S, Storck W, Hobernik D, Raker VK, Fischer K, Decker S, Dzionek A, Krauthäuser S, Diken M, Nikolaev A, Maxeiner J, Schuster P, Kappel C, Verschoor A, Schild H*, Grabbe S*, Bros M*. Protein corona-mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses. (2018) J Allergy Clin Immunol. 142(5):1558-1570.
Nuhn L, De Koker S, Van Lint S, Zhong Z, Catani JP, Combes F, Deswarte K, Li Y, Lambrecht BN, Lienenklaus S, Sanders NN, David SA*, Taverrnier J*, De Geest BG*. Nanoparticle-conjugated TLR7/8 agonist localized immunotherapy provokes safe and synergistic antitumoral responses. (2018) Adv Mater. 30(45):1803397.
Weber B, Kappel C, Scherer M, Helm M, Bros M, Grabbe S, Barz M. PeptoSomes for vaccination: combining antigen and adjuvant in polypept(o)id-based polymersomes. (2017) Macromol Biosci. 17(10).
This project will develop DNA-vaccines, which are under the translational control of the Fascin-promotor and thus only active in dendritic cells. Since dendritic cells as – non-dividing cells – are rather difficult to transfect, the expertise of E. Wagner and R. Zentel will be combined to develop new, more efficient stimulus responsive transfection systems from blockcopolymers and cationic oligomers.
M. Bros, E. Wagner, R. Zentel
Project relevant publications
Hager S, Fittler FJ, Wagner E, Bros M. Nucleic acid-based approaches for tumor therapy. (2020) MDPI Cells.
Ritt N, Berger S, Wagner E, Zentel R. Versatile, Multifunctional Block-Copolymers for the Self-Assembly of Well-Defined, Non-Toxic pDNA Polyplexes. (2020) ACS Applied Polymer Materials.
Wagener K, Bros M, Krumb, M, Langhanki J, Pektor S, Worm M, Schinnerer M, Montermann E, Miederer M, Frey H, Opatz T, Rösch F. Targeting of Immune Cells with Trimannosylated Liposomes. (2020) Advanced Therapeutics. 3:1900185.
Kramer S, Langhanki J, Krumb M, Opatz T, Bros M, Zentel R. HPMA-Based Nanocarriers for Effective Immune System Stimulation. (2019) Macromol Biosci. 19(6):e1800481.
Klein PM, Kern S, Lee DJ, Schmaus J, Höhn M, Gorges J, Kazmaier U, Wagner E. Folate receptor-directed orthogonal click-functionalization of siRNA lipopolyplexes for tumor cell killing in vivo. (2018) Biomaterials.
Reinhard S, Wang Y, Dengler S, Wagner E. Precise Enzymatic Cleavage Sites for Improved Bioactivity of siRNA Lipo-Polyplexes. (2018) Bioconjug Chem. 29:3649-3657.
Shen L, Tenzer S, Storck W, Hobernik D, Raker VK, Fischer K, Decker S, Dzionek A, Krauthäuser S, Diken M, Nikolaev A, Maxeiner J, Schuster P, Kappel C, Verschoor A, Schild H, Grabbe S, Bros M. Protein corona-mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses. (2018) J Allergy Clin Immunol. 142(5):1558-1570.
Moog KE, Barz M, Bartneck M, Beceren-Braun F, Mohr N, Wu Z, Braun L, Dernedde J, Liehn EA, Tacke F, Lammers T, Kunz H, Zentel R. Polymeric Selectin Ligands Mimicking Complex Carbohydrates: From Selectin Binders to Modifiers of Macrophage Migration. (2017) Angew. Chem. Int. Ed. 56(5):1416-421.
Krhac Levacic A, Morys S, Kempter S, Lächelt U, Wagner E. Minicircle Versus Plasmid DNA Delivery by Receptor-Targeted Polyplexes. (2017) Human Gene Ther. 28(10).
Heller P, Hobernik D, Lächelt U, Schinnerer M, Weber B, Schmidt M, Wagner E, Bros M, Barz M. Combining reactive triblock copolymers with functional cross-linkers: A versatile pathway to disulfide stabilized-polyplex libraries and their application as pDNA vaccines. (2017) J Control Release. 258:146-160.
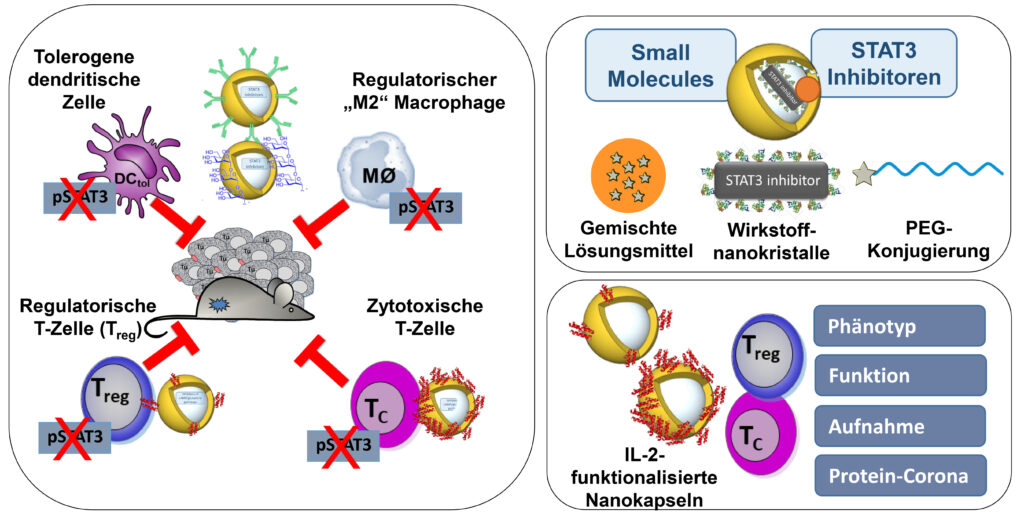
STAT3-mediated tumor-associated processes will be modulated by use of STAT3 inhibitor loaded functionalized nanocapsules. We will analyse the impact on tolerogenic DC and myeloid cells and, in particular, on T cells (regulatory FOXP3+, cytotoxic CD8+ T cells), thereby taking advantage of the established method of IL-2 functionalized nanocapsules for T cell targeting. Further studies will unravel the therapeutical effect of our nanocapsule-based systems (+/- immunotherapies) in murine and humanized [MISTRG] models of malignant melanoma in vivo. in vivo getestet und mit weiteren Immuntherapien kombiniert.
K. Landfester, K. Steinbrink
Project relevant publications
Frey ML, Simon J, Brückner M, Mailänder V, Morsbach S, Landfester K. Bio-orthogonal triazolinedione (TAD) crosslinked protein nanocapsules affect protein adsorption and cell interaction. Polym Chem. 2020, 11, 3821–383.
Raker V, Becker C, Landfester K, Steinbrink K. Targeted activation of T cells with IL-2-coupled nanoparticles. Cells. 2020, 9, 9, E2063.
Gai M, Simon J, Lieberwirth I, Mailänder V, Morsbach S, Landfester K. A bio-orthogonal functionalization strategy for site-specific coupling of antibodies on vesicle surfaces after self-assembly. Polym. Chem. 2020, 11, 527-540.
Stegemann A, Flis D, Ziolkowski W, Distler JHW, Steinbrink K, Böhm M, Stegemann A. The α7 nicotinic acetylcholine receptor: a promising target for the treatment of fibrotic skin disorders. J Invest Dermatol. 2020; 23:S0022-202X(20)31399-3.
Thiramanas R, Jiang S, Simon J, Landfester K, Mailänder V. Silica Nanocapsules with Different Sizes and Physicochemical Properties as Suitable Nanocarriers for Uptake in T-Cells. Intern. J. Nanomedicine 2020, 15, 6069–6084
Haub J, Roehrig N, Uhrin P, Schabbauer G, Eulberg D, Melchior F, Shahneh F, Probst HC, Becker C, Steinbrink K*, Raker VK* Intervention of inflammatory monocyte activity limits dermal fibrosis. J Invest Dermatol. 2019, 139, 2144-2153. *equal contribution
Guindani C, Frey ML, Simon J, Koynov K, Schultze J, Ferreira SRS, Araujo PHH, Oliveira D, Wurm FR, Mailänder V, Landfester K. Covalently Binding of Bovine Serum Albumin to UnsaturatedPoly(Globalide-Co-epsilon-Caprolactone) Nanoparticles by Thiol-Ene Reactions, Macromol Biosci, 2019, 19(10), e1900145.
Kleszczyński K, Kim TK, Bilska B, Sarna M, Mokrzynski K, Stegemann A, Pyza E, Reiter RJ, Steinbrink K, Böhm M, Slominski AT, Kleszczyński K. Melatonin exerts oncostatic capacity and decreases melanogenesis in human MNT-1 melanoma cells. J Pineal Res. 2019; 67(4):e12610.
Agrawalla BK, Wang T, Riegger A, Domogalla MP, Steinbrink K, Dörfler T, Chen X, Boldt F, Lamla M, Michaelis J, Kuan SL, Weil T. Chemoselective dual labeling of native and recombinant proteins. Bioconjug Chem. 2018; 29: 29-34.
Schmidt T, Lorenz N, Raker VK, Schmidgen MI, Mahnke K, Enk A, Roth J, Steinbrink K. Allergen-specific low zone tolerance is independent of MRP8/14-, TLR4-, TLR7-, and TLR9-mediated immune processes. J Invest Dermatol. 2018; 138: 452-455.
Tonigold M, Simon J, Estupinan D, Kokkinopoulou M, Reinholz J, Kintzel U, Kaltbeitzel A, Renz P, Domogalla MP, Steinbrink K, Lieberwirth I, Crespy D, Landfester K, Mailänder V. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. Nat Nanotechnol. 2018, 13, 862-869.
Passlick D, Piradashvili K, Bamberger D, Li M, Jiang S, Strand D, Wich P, Landfester K, Bros M, Grabbe S, Mailänder V. Delivering all in one: Antigen-nanocapsule loaded with dual adjuvant yields superadditive effects by DC-directed T cell stimulation, J. Contr. Release 2018, 289, 23-34
Alkanawati MS, Wurm FR, Therien-Aubin H, Landfester K. Large-Scale Preparation of Polymer Nanocarriers by High-Pressure Microfluidization. Macromol Mater Eng. 2018, 303, Art. No. 1700505
Haeberle S, Raker V, Haub J, Kim YO, Weng SY, Yilmaz OK, Enk A, Steinbrink K*, Schuppan D*, Hadaschik EN.* Regulatory T cell deficient scurfy mice exhibit a Th2/M2-like inflammatory response in the skin. J Dermatol Sci. 2017, 87, 285-291. *equal contribution
Piradashvili K, Simon J, Passlick D, Hoehner JR, Mailänder V, Wurm FR, Landfester K. Fully degradable protein nanocarriers by orthogonal photoclick tetrazole-ene chemistry for the encapsulation and release. Nanoscale Horizons. 2017, 2, 297-302.
Domogalla MP, Rostan PV, Raker VK, Steinbrink K. Tolerance through education: how tolerogenic dendritic cells shape immunity. Front Immunol. 2017; 11;8:1764. doi: 10.3389/fimmu.2017.01764.
Raker V, Haub J, Stojanovic A, Cerwenka A, Schuppan D, Steinbrink K. Early inflammatory players in cutanous fibrosis. J Dermatol Sci. 2017; 87:228-235.
Müller, J.; Bauer, KN.; Prozeller, D.; Simon, J.; Mailander, V.; Wurm, F.R.; Winzen, S.; Landfester, K. Coating nanoparticles with tunable surfactants facilitates control over the protein corona. Biomaterials. 2017, 115, 1-8.
We have shown that adenylyl cyclase inhibitor-loaded polypept(o)ide micelles suppress the growth of melanomas by repressing intratumoral cAMP formation. In preparation of a clinical trial, the effect of particle-mediated cAMP repression on human melanomas and syngeneic human immune cells will now be investigated in the humanized MISTRG mouse melanoma model. We aim to to make micelle-mediated cAMP repression by adjuvant nanoparticles even more effective and to identify the involved signal transduction pathways in tumor-infiltrating leukocytes by comparative transcriptome analyses.
M. Barz, C. Becker, T. Bopp
Project relevant publications
Dal NK, Kocere A, Wohlmann J, Van Herck S, Bauer TA, Resseguier J, Bagherifam S, Hyldmo H, De Geest BG, Barz M, Fenaroli F. Zebrafish Embryos Allow Prediction of Nanoparticle Circulation Times in Mice and Facilitate Quantification of Nanoparticle-Cell Interactions. (2020) Small. 1906719.
Bohn T, Rapp S, Luther N, Klein M, Bruehl TJ, Kojima N, Aranda Lopez P, Hahlbrock J, Muth S, Endo S, Pektor S, Brand A, Renner K, Popp V, Gerlach K, Vogel D, Lueckel C, Arnold-Schild D, Pouyssegur J, Kreutz M, Huber M, Koenig J, Weigmann B, Probst HC, von Stebut E, Becker C, Schild H, Schmitt E, and Bopp T. Tumor immunoevasion via acidosis dependent induction of regulatory tumor-associated macrophages. (2018) Nature Immunology. 19(12):1319-1329.
Fenaroli F, Repnik U, Xu Y, Johann K, Van Herck S, Dey P, Skjeldal FM, Frei DM, Bagherifam S, Kocere A, Haag R, De Geest BG, Barz M, Griffiths G. Enhanced permeability and retention-like extravasation of nanoparticles from the vasculature into tuberculosis granulomas in zebrafish and mouse models. (2018) ACS nano. 12(8):8646-8661.
Muhl C, Schäfer O, Bauer T, Räder HJ, Barz M. Poly(S-ethylsulfonyl-l-homocysteine): An α-Helical Polypeptide for Chemoselective Disulfide Formation. (2018) Macromolecules. 51(20):8188-8196.
Schäfer O, Klinker K, Braun L, Huesmann D, Schultze J, Koynov K, Barz M. Combining orthogonal reactive groups in block copolymers for functional nanoparticle synthesis in a single step. (2017) ACS Macro Letters. 6(10):1140-1145.
Klinker K, Schäfer O, Huesmann D, Bauer T, Capelôa L, Braun L, Stergiou N, Schinnerer M, Dirisala A, Miyata K, Osada K, Cabral H, Kataoka K, Barz M. Secondary-Structure-Driven Self-Assembly of Reactive Polypept(o)ides: Controlling Size, Shape, and Function of Core-Crosslinked Nanostructures. (2017) Angew. Chem. Intern. Ed. 56(32):9608-9613.
Birke A, Huesmann D, Kelsch A, Weilbächer M, Xie J, Bros M, Bopp T, Becker C, Landfester K, Barz M. Polypeptoid-block-polypeptide copolymers: synthesis, characterization, and application of amphiphilic block Copolypept(o)ides in drug formulations and miniemulsion techniques. (2014) Biomacromolecules. 15(2):548-57.
Bacher N, Raker V, Hofmann C, Graulich E, Schwenk M, Baumgrass R, Bopp T, Zechner U, Merten L, Becker C, Steinbrink K. Interferon-α suppresses cAMP to disarm human regulatory T cells. (2013) Cancer Res. 73(18):5647-56.
Becker C, Bopp T, Steinbrink K. Interferon α interferes with immunological tolerance. (2013) Oncoimmunology. 2(12):e27528.
Vaeth M*, Gogishvili T*, Bopp T*, Klein M, Berberich-Siebelt F, Gattenloehner S, Avots A, Sparwasser T, Grebe N, Schmitt E, Hünig T, Serfling E, Bodor J. Regulatory T cells facilitate the nuclear accumulation of inducible cAMP early repressor (ICER) and suppress nu-clear factor of activated T cell c1 (NFATc1). (2011) Proc Natl Acad Sci U S A. 108(6):2480-5. *Joint first authors
Starting with the observation that some nanocarriers build up a protein corona nearly instantaneously in context with serum proteins, which has serious results for cellular uptake and intra-cellular trafficking, this project aims at three different topics. At first it will study possible corona formation on more carrier systems to determine the generality of this phenomenon. Then the applicants want to look for ways to pre-organize desired proteins in the corona to modify biodistribution. At last it is the aim to bind desired proteins like clusterin and/or antibodies chemically to obtain –in this way- a stealth like behavior.
V. Mailänder, S. Tenzer, T. Weil
Project relevant publications
Alberg I, Kramer S, Schinnerer M, Hu Q, Seidl C, Leps C, Drude N, Möckel D, Rijcken C, Lammers T, Diken M, Maskos M, Morsbach S, Landfester K, Tenzer S*, Barz M*, Zentel R*. Polymeric Nanoparticles with Neglectable Protein Corona. (2020) Small. 16(18):e1907574.* Corresponding authors
Xu L, Raabe M, Zegota MM, Nogueira J, Chudasama V, Kuan SL, Weil T. Site-selective protein modification via disulfide rebridging for fast tetrazine/trans-cyclooctene bioconjugation. (2020) Org. Biomol. Chem.
Wagner J, Li L, Simon J, Krutzke L, Müllen K, Landfester K, Mailänder V, Ng DYW, Wu YZ, Weil T. Amphiphilic Polyphenylene Dendron Conjugates for Surface Remodeling of Adenovirus 5. (2020) Angewandte Chemie International Edition. 59(14):5712-5720. Angewandte Chemie. 132(14):5761-5769.
Wagner J, Dillenburger M, Simon J, Oberländer J, Landfester K, Mailänder V, Ng DYW, Müllen K, Weil T. Amphiphilic Dendrimers Control Protein Binding and Corona Formation on Liposome Nanocarriers. (2020) Chemical Communications. 56(61):8663-8666.
Weber C, Voigt M, Simon J, Danner AK, Frey H, Mailänder V, Helm M, Morsbach S, Landfester K. Functionalization of Liposomes with Hydrophilic Polymers Results in Macrophage Uptake Independent of the Protein Corona. (2019) Biomacromolecules. 20(8):2989-2999.
Distler U, Łącki MK, Schumann S, Wanninger M, Tenzer S. Enhancing Sensitivity of Microflow-Based Bottom-Up Proteomics through Postcolumn Solvent Addition. (2019) Anal Chem.. 91(12):7510-7515.
Prozeller D, Pereira J, Simon J, Mailänder V, Morsbach S, Landfester K. Prevention of Dominant IgG Adsorption on Nanocarriers in IgG-Enriched Blood Plasma by Clusterin Precoating. (2019) Adv Sci. 6(10):1802199.
Tonigold M, Simon J, Estupinan D, Kokkinopoulou M, Reinholz J, Kintzel U, Kaltbeitzel A, Renz P, Domogalla MP, Steinbrink K, Lieberwirth I, Crespy D, Landfester K, Mailänder V. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. (2018) Nature Nanotechnology. 13(9):862-869.
Shen L, Tenzer S, Storck W, Hobernik D, Raker VK, Fischer K, Decker S, Dzionek A, Krauthäuser S, Diken M, Nikolaev A, Maxeiner J, Schuster P, Kappel C, Verschoor A, Schild H, Grabbe S, Bros M. Protein corona-mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses. (2018) J Allergy Clin Immunol. 142(5):1558-1570.
Zegota MM, Wang T, Seidler C, Ng DYW, Kuan SL, Weil T. “Tag and Modify” Protein Conjugation with Dynamic Covalent Chemistry. (2018) Bioconjugate Chemistry. 29(8):2665-2670.
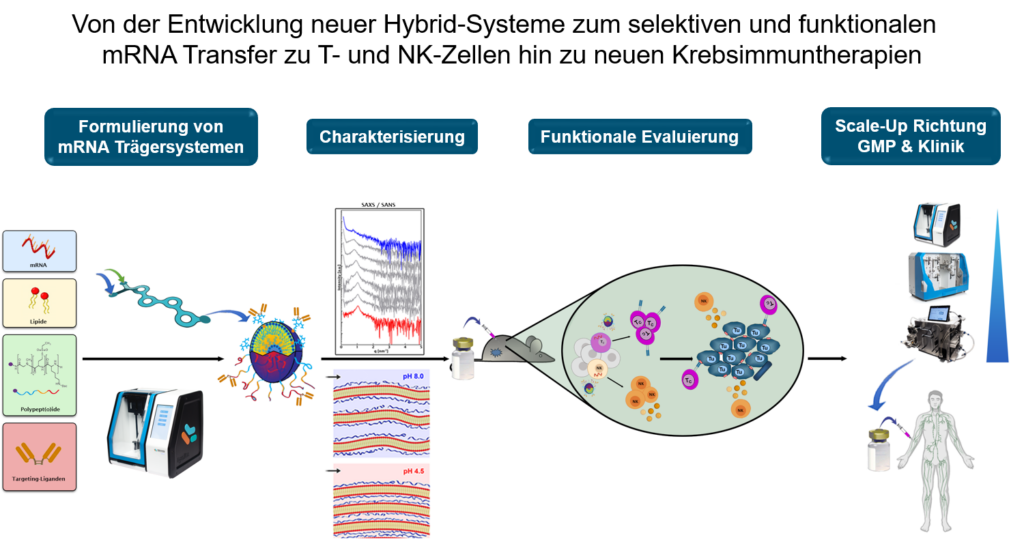 Aim of TP B12 is the development of medically applicable nanoparticular delivery systems to allow functional modulation of T- and NK-cells by mRNA-coding cytokines. RNA-Lipoplex-NP initially developed by Sahin et al. are already investigated in clinical studies for the treatment of melanoma. To further expand the concept of mRNA based immune therapies onto T- and NK-cells polymer-lipid-hybrid and ligand systems were established and will be further developed regarding their clinical translation for immune therapies of melanoma.
Aim of TP B12 is the development of medically applicable nanoparticular delivery systems to allow functional modulation of T- and NK-cells by mRNA-coding cytokines. RNA-Lipoplex-NP initially developed by Sahin et al. are already investigated in clinical studies for the treatment of melanoma. To further expand the concept of mRNA based immune therapies onto T- and NK-cells polymer-lipid-hybrid and ligand systems were established and will be further developed regarding their clinical translation for immune therapies of melanoma.
M. Barz, P. Langguth, U. Sahin
Project relevant publications
Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D, Kuhn AN, Omokoko T, Kranz LM, Diken M, Kreiter S, Haas H, Attig S, Rae R, Cuk K, Kemmer-Brück A, Breitkreuz A, Tolliver C, Caspar J, Quinkhardt J, Hebich L, Stein M, Hohberger A, Vogler I, Liebig I, Leierer M, Müller V, Mitzel-Rink H, Miederer M, Huber C, Grabbe S, Utikal J, Pinter A, Kaufmann R, Hassel JC, Loquai C, Türeci Ö. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. (2020) Nature. 585(7823):107-112.
Nogueira S, Schlegel A, Maxeiner K, Weber B, Barz M, Schroer M A, Blanchet C E, Svergun D, Ramishetti S, Peer D, Langguth P, Sahin U, Haas H. Polysarcosine-Functionalized Lipid Nanoparticles for Therapeutic mRNA Delivery. (2020) ACS Applied Nano Materials. DOI: 10.1021/acsanm.Oc01834.
Siewert C, Haas H, Cornet V, Nogueira SS, Nawroth T, Uebbing L, Ziller A, Al-Gousous J, Radulescu A, Schroer MA, Blanchet CE, Svergun DI, Radsak MP, Sahin U, Langguth P. Hybrid Biopolymer and Lipid Nanoparticles with Improved Transfection Efficacy for mRNA. (2020) Cells. 9(9): 2034.
Uebbing L, Ziller A, Siewert C, Schroer MA, Clement BE, Svergun DI, Ramishetti S, Peer D, Sahin U, Haas H, Langguth P. Investigation of pH-responsiveness inside lipid nanoparticles for parenteral mRNA application using small angle X-ray scattering. (2020) Langmuir. DOI: 10.1021/acs.langmuir.0c02446.
Siewert C, Haas H, Nawroth T, Ziller A, Nogueira SS, Schroer MA, Blanchet CE, Svergun DI, Radulescu A, Bates F, Huesemann Y, Radsak MP, Sahin U, Langguth P. Investigation of charge ratio variation in mRNA – DEAE-dextran polyplex delivery systems. (2019) Biomaterials. 192:612-620
Sun Q, Barz M, De Geest BG, Diken M, Hennink WE, Lammers T, Shi Y. Nanomedicine and macroscale materials in immuno-oncology. (2019) Chem. Soc. Rev. 48(1):351-381.
Bleher S, Buck J, Muhl C, Sieber S, Barnert S, Witzigmann D, Huwyler J, Barz M, Süss R. Poly(Sarcosine) Surface Modification Imparts Stealth-Like Properties to Liposomes. (2019) Small. 15(50):e1904716.
Ziller A, Nogueira SS, Hühn E, Funari SS, Brezesinski G, Hartmann H, Sahin U, Haas H, Langguth P. Incorporation of mRNA in Lamellar Lipid Matrices for Parenteral Administration. (2018) Molecular pharmaceutics. 15(2):642-651.
Heller P, Zhou J, Weber B, Hobernik D, Bros M, Schmid F, Barz M. The Influence of Block Ionomer Microstructure on Polyplex Properties: Can Simulations Help to Understand Differences in Transfection Efficiency? (2017) Small. 13(17).
Heller P, Hobernik D, Lächelt U, Schinnerer M, Weber B, Schmidt M, Wagner E, Bros M, Barz M. Combining reactive triblock copolymers with functional cross-linkers: A versatile pathway to disulfide stabilized-polyplex libraries and their application as pDNA vaccines. (2017) Journal of controlled release. 258:146-160.
TP B13 established a modular vaccine platform using molecular (glyco)peptide building blocks. These carry tumor-associated antigens or immuno-stimulatory components and co-assemble into anisotropic multifunctional nanorods. The platform was successfully applied as fully synthetic vaccines against tumor-associated Mucin 1 during the previous funding period and will now be used to develop vaccines against malignant melanoma. Synthetic B-cell epitopes derived from melanoma associated surface protein CSPG4 will be co-presented on the nanorod surface, with T-cell epitopes and immunomodulating agents, and will be evaluated in vivo in terms of tumor-specific antibody induction and therapeutic effect.
P. Besenius, T. Bopp
Project relevant publications
Stergiou N, Nagel J, Pektor S, Heimes AS, Jäkel J, Brenner W, Schmidt M, Miederer M, Kunz H, Rösch F, Schmitt E. Evaluation of a novel monoclonal antibody against tumor-associated MUC1 for diagnosis and prognosis of breast cancer. (2019) Int J Med Sci. 16:1188-1198.
Stergiou N, Gaidzik N, Heimes AS, Dietzen S, Besenius P, Jäkel J, Brenner W, Schmidt M, Kunz H, Schmitt E. Reduced Breast Tumor Growth After Immunization with a Tumor-Restricted MUC1 Glycopeptide Conjugated to Tetanus Toxoid. (2019) Cancer Immunol Res. 7:113-122.
Straßburger D, Glaffig M, Stergiou N, Bialas S, Besenius P, Schmitt E, Kunz H. Synthetic MUC1 Antitumor Vaccine with Incorporated 2,3-Sialyl-T Carbohydrate Antigen Inducing Strong Immune Responses with Isotype Specificity. (2018) ChemBioChem. 19:1142-1146.
Straßburger D, Stergiou N, Urschbach M, Yurugi H, Spitzer D, Schollmeyer D, Schmitt E, Besenius P. Mannose-Decorated Multicomponent Supramolecular Polymers Trigger Effective Uptake into Antigen-Presenting Cells. (2018) ChemBioChem. 19:912-916.
Glaffig M, Stergiou N, Hartmann S, Schmitt E, Kunz H. A Synthetic MUC1 Anticancer Vaccine Containing Mannose Ligands for Targeting Macrophages and Dendritic Cells. (2018) ChemMedChem. 13:25-29.
Stergiou N, Glaffig M, Jonuleit H, Kunz H, Schmitt E. Immunization With a Synthetic Human MUC1 Glycopeptide Vaccine Against Tumor-Associated MUC1 Breaks Tolerance in Human MUC1 Transgenic Mice. (2017) ChemMedChem. 12:1424-1428.
Spitzer D, Rodrigues LL, Straßburger D, Mezger M, Besenius P. Tuneable Transient Thermogels Mediated by a pH- and Redoxregulated Supramolecular Polymerization. (2017) Angew. Chem. Int. Ed. 56:15461-15465.
Jonuleit H, Bopp T, Becker C. Treg cells as potential cellular targets for functionalized nanoparticles in cancer therapy. (2016) Nanomedicine. 11:2699-2709.
Frisch H, Nie Y, Raunser S, Besenius P. pH-Regulated Selectivity in Supramolecular Polymerizations: Switching Between Co- and Homopolymers. (2015) Chem. Eur. J. 21:3304-3309.
Weilbächer M, Allmeroth M, Hemmelmann M, Ritz S, Mailänder V, Bopp T, Barz M, Zentel R. Interaction of N-(2-hydroxypropyl)methacrylamide based homo, random and block copolymers with primary immune cells. (2014) J Biomed Nanotechnol. 10:81–91.
In a humanized mouse model of malignant melanoma, immunomodulation in tumor tissue is addressed, in particular by targeting inhibitory cell populations. pH-responsive liposomes from the previous funding period are further developed into smart, programmable liposomes with time-programmable release and pH-modulating properties in order to selectively release active substances combined with pH-modulation of the tumor microenvironment for intervention in the signaling pathways of mainly macrophages, regulatory T cells (Treg) and tumor cells.
M. Helm, A. Tüttenberg, A. Walther
Projektrelevante Publikationenseit 2017:
Jonathan Schupp, Arne Christians, Niklas Zimmer, Lukas Gleue, Helmut Jonuleit, Mark Helm and Andrea Tuettenberg. In depth immune-oncology studies of the tumor microenvironment in a humanized melanoma mouse model. In press, IJMS 2021.
Krebs FK, Trzeciak E, Zimmer S, Oezistanbullu D, Mitzel-Rink H, Meissner M, Grabbe S, Loquai C, Tuettenberg A. Immune signature as predictive marker for response to checkpoint inhibitor immunotherapy and overall survival in melanoma. Cancer Med. (2021). DOI:10.1002/cam4.3710.
Zimmer N, Krebs FK, Zimmer S, Mitzel-Rink H, Kumm EJ, Jurk K, Grabbe S, Loquai C, Tuettenberg A. Platelet-Derived GARP Induces Peripheral Regulatory T Cells-Potential Impact on T Cell Suppression in Patients with Melanoma-Associated Thrombocytosis. Cancers (Basel). (2020) Dec 5;12(12):3653. doi: 10.3390/cancers12123653.
Schupp J, Krebs FK, Zimmer N, Trzeciak E, Schuppan D, Tuettenberg A. Targeting myeloid cells in the tumor sustaining microenvironment. (2019) Cell Immunol. 343:103713.
Zimmer N, Kim E, Sprang B, Leukel P, Khafaji F, Ringel F, Sommer C, Tuettenberg J, Tuettenberg A. GARP as an immune regulatory molecule in the tumormicroenvironment of glioblastoma multiforme. (2019) Int J Mol Sci. 20: 3676.
Weber C, Voigt M, Simon J, Danner AK, Frey H, Mailänder V, Helm M, Morsbach S, Landfester K. Functionalization of Liposomes with Hydrophilic Polymers Results in Macrophage Uptake Independent of the Protein Corona. (2019) Biomacromolecules. 20:2989-2999.
Leber N, Kaps L, Aslam M, Schupp J, Brose A, Schäffel D, Fischer K, Diken M, Strand D, Koynov K, Tuettenberg A, Nuhn L, Zentel R, Schuppan D. SiRNA-mediated in vivo gene knockdown by acid-degradable cationic nanohydrogel particles. (2017) J Control Release. 248:10-23.
Heinen L, Heuser T, Steinschulte A, Walther A. Antagonistic Enzymes in a Biocatalytic pH Feedback System Program Autonomous DNA Hydrogel Life Cycles. (2017) Nano Lett. 17:4989.
Hellmuth I, Freund I, Schlöder J, Seidu-Larry S, Thüring K, Slama K, Langhanki J, Kaloyanova S, Eigenbrod T, Krumb M, Röhm S, Peneva K, Opatz T, Jonuleit H, Dalpke AH, Helm M. Bioconjugation of Small Molecules to RNA Impedes Its Recognition by Toll-Like Receptor. (2017) Front Immunol. 8:312.
Hahn SA, Neuhoff A, Landsberg J, Schupp J, Eberts D, Leukel P, Bros M, Weilbaecher M, Schuppan D, Grabbe S, Tueting T, Lennerz V, Sommer C, Jonuleit H, Tuettenberg A. A key role of GARP in the immune suppressive tumor microenvironment. (2016) Oncotarget. 7:42996-43009.
Fritz T, Voigt M, Worm M, Negwer I, Müller SS, Kettenbach K, Ross TL, Roesch F, Koynov K, Frey H, Helm M. Orthogonal Click Conjugation to the Liposomal Surface Reveals the Stability of the Lipid Anchorage as Crucial for Targeting. (2016) Chem. Eur. J. 22:11578-82.
Heuser T, Steppert AK, Molano-Lopez C, Zhu B, Walther A. A Generic Concept to Program the Time Domain of Self-Assemblies with a Self-Regulation Mechanism. (2015) Nano Lett. 15:2213.
Heuser T, Weyandt E, Walther A. Biocatalytic Feedback-Driven Temporal Programming of Self-Regulating Non-Equilibrium Peptide Hydrogels. (2015) Angew. Chem. Int. Ed. 54:13258.
Nanovaccines accumulate in the liver typically in tolerance-promoting KC (Kupffer cells) and LSEC (sinusoidal liver endothelial cells). Both cell types can be reprogrammed to serve as antigen presenting cells by applying suitable adjuvants. B15N develops nanovaccines which besides dendritic cells also address and activate KC/LSEC. This approach serves to induce potent anti-tumor responses in liver and skin (metastatic melanoma). The suitability of protein nanocapsules containing peptid antigen/adjuvant and of liposomes loaded with antigen-coding mRNA/nucleic acid-based adjuvants to serve as nanovaccines will be evaluated.
M. Bros, S. Gehring, K. Landfester
Project relevant publications
Cacicedo ML, Medina-Montano C, Kaps L, Kappel C, Gehring S, Bros M. Role of Liver-Mediated Tolerance in Nanoparticle-Based Tumor Therapy. (2020) Cells. 9(9):1985.
Wagener K, Bros M, Krumb M, Langhanki J, Pektor S, Worm M, Schinnerer M, Montermann E, Miederer M, Frey H, Opatz T, Rösch F. Targeting of Immune Cells with Trimannosylated Liposomes. (2020) Advanced Therapeutics. 3(6):1900185.
Paßlick D, Piradashvili K, Bamberger D, Li M, Jiang S, Strand D, R. Wich P, Landfester K, Bros M, Grabbe S, Mailänder V. Delivering all in one: Antigen-nanocapsule loaded with dual adjuvant yields superadditive effects by DC-directed T cell stimulation. (2018) Journal of Controlled Release. 289:23-34.
Shen L, Tenzer S, Storck W, Hobernik D, Raker VK, Fischer K, Decker S, Dzionek A, Krauthäuser S, Diken M, Nikolaev A, Maxeiner J, Schuster P, Kappel C, Verschoor A, Schild H, Grabbe S, Bros M. Protein corona–mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses. (2018) Journal of Allergy and Clinical Immunology. 142(5):1558-1570.
Bros M, Nuhn L, Simon J, Moll L, Mailänder V, Landfester K, Grabbe S. The Protein Corona as a Confounding Variable of Nanoparticle-Mediated Targeted Vaccine Delivery. (2018) Frontiers in Immunology. 9:1760.
Fichter M, Piradashvili K, Pietrzak-Nguyen A, Pretsch L, Kuhn G, Strand S, Knuf M, Zepp F, Wurm FR, Mailänder V, Landfester K, Gehring S. Polymeric hepatitis C virus non-structural protein 5A nanocapsules induce intrahepatic antigen-specific immune responses. (2016) Biomaterials. 108:1-12.
Pietrzak-Nguyen A, Piradashvili K, Fichter M, Pretsch L, Zepp F, Wurm FR, Landfester K, Gehring S. MPLA-coated hepatitis B virus surface antigen (HBsAg) nanocapsules induce vigorous T cell responses in cord blood derived human T cells. (2016) Nanomedicine: Nanotechnology, Biology and Medicine. 12(8):2383-2394.
Piradashvili K, Fichter M, Mohr K, Gehring S, Wurm FR, Landfester K. Biodegradable Protein Nanocontainers. (2015) Biomacromolecules. 16(3):815-821.
Fichter M, Dedters M, Pietrzak-Nguyen A, Pretsch L, Meyer CU, Strand S, Zepp F, Baier G, Landfester K, Gehring S. Monophosphoryl lipid A coating of hydroxyethyl starch nanocapsules drastically increases uptake and maturation by dendritic cells while minimizing the adjuvant dosage. (2015) Vaccine. 33(7):838-846.
Pietrzak-Nguyen A, Fichter M, Dedters M, Pretsch L, Gregory SH, Meyer C, Doganci A, Diken M, Landfester K, Baier G, Gehring S. Enhanced in Vivo Targeting of Murine Nonparenchymal Liver Cells with Monophosphoryl Lipid A Functionalized Microcapsules. (2014) Biomacromolecules. 15(7):2378-2388.
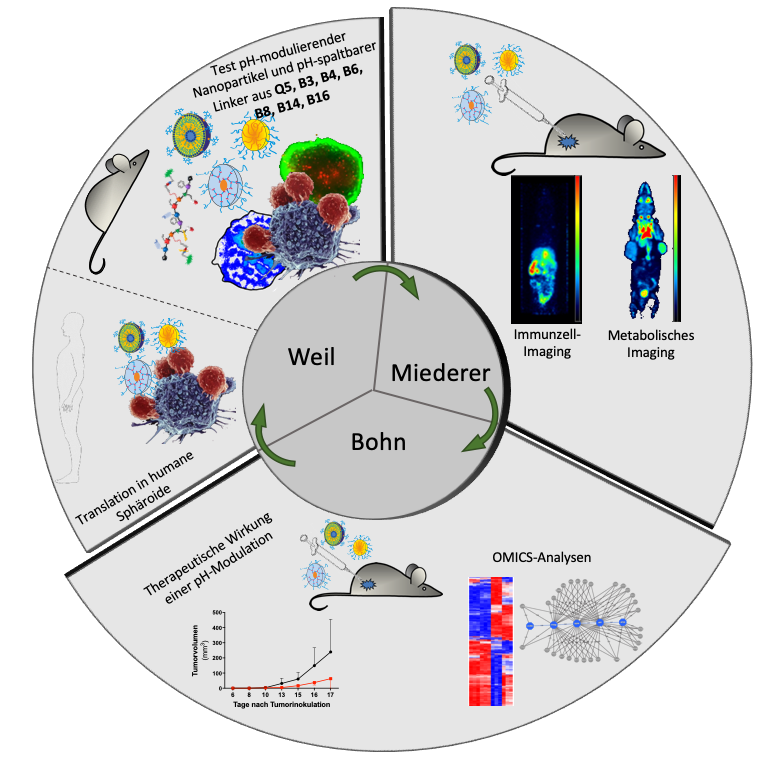
The goal of B16N is to change the metabolic status of the tumor microenvironment using pH-modulating nanocarriers. Aggressive, fast-growing tumors are characterized by an acidic microenvironment that pro-motes the differentiation of a non-inflammatory macrophage subtype. The increase of the pH value will be imaged using PET and the resulting effects on tumor infiltrating immune cells will be investigated using PET and OMICS analyses. Furthermore, tumor spheroids generated from human tumor biopsies are used to translate pH modulation and its effects on immune cell infiltrates to human tumors.
T. Bohn, M. Miederer, T. Weil
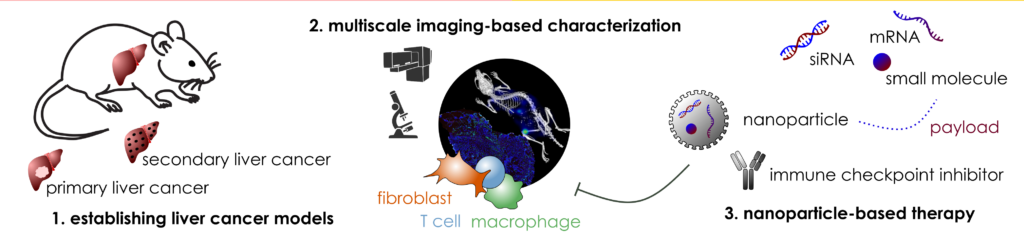
Nanoparticles are decorated with CAF-targeting ligands, loaded with CAF-modulating drugs (e.g. anti-TGFβ1 siRNA, AT1R-antagonists) and tested in vitro (in i.a. tumor organoids) to select promising candidates for therapeutic in vivo experiments. After biodistribution and tumor tissue accumulation of the drug-loaded nanoparticles have been assessed by multimodal imaging methods, therapeutic antitumor effects of the validated drug-loaded nanoparticles are studied in relevant murine liver tumor models, whereby i.a. RNA- and proteomics are applied to gain insight into therapeutic mechanisms.
M. Diken, L. Kaps, T. Lammers
Project relevant publications
May JN, Golombek SK, Baues M, Dasgupta A, Drude N, Rix A, Rommel D, Von Stillfried S, Appold L, Pola R, Pechar M, Van Bloois L, Storm G, Kuehne AJC, Gremse F, Theek B, Kiessling F, Lammers T. Multimodal and multiscale optical imaging of nanomedicine delivery across the blood-brain barrier upon sonopermeation. (2020) Theranostics. 10:1948-1959.
Wang B, Van Herck S, Chen Y, Bai X, Zhong Z, Deswarte K, Lambrecht BN, Sanders NN, Lienenklaus S, Scheeren HW, David SA, Kiessling F, Lammers T*, De Geest BG*, Shi Y*. Potent and Prolonged Innate Immune Activation by Enzyme-Responsive Imidazoquinoline TLR7/8 Agonist Prodrug Vesicles. (2020) J Am Chem Soc. 142:12133-12139.
Sun Q, Baues M, Klinkhammer BM, Ehling J, Djudjaj S, Drude NI, Daniel C, Amann K, Kramann R, Kim H, Saez-Rodriguez J, Weiskirchen R, Onthank DC, Botnar RM, Kiessling F, Floege J, Lammers T*, Boor P*. Elastin imaging enables noninvasive staging and treatment monitoring of kidney fibrosis. (2019) Sci Transl Med. 11:eaat4865.
Sun Q, Barz M, De Geest BG, Diken M, Hennink WE, Kiessling F, Lammers T*, Shi Y*. Nanomedicine and macroscale materials in immuno-oncology. (2019) Chem Soc Rev. 48:351-381.
Kaps L*, Leber N*, Yang A, Aslam M, Giardino M, Klefenz A, Choteschovsky N, Rosigkeit S, Mostafa A, Nuhn L, Schuppan D, Zentel R. α-Mannosyl-Functionalized Cationic Nanohydrogel Particles for Targeted Gene Knockdown in Immunosuppressive Macrophages. (2019) Macromol Biosci. 1900162:1900162.
van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. (2019) Nat Nanotechnol. 14:1007-1017.
Kaps L*, Leber N*, Aslam M, Schupp J, Brose A, Schäffel D, Fischer K, Diken M, Strand D, Koynov K, Tuettenberg A, Nuhn L, Zentel R, Schuppan D. SiRNA-mediated in vivo gene knockdown by acid-degradable cationic nanohydrogel particles. (2017) J Control Release. 248:10-23.
Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Müller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Brück AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Höller C, Utikal J, Huber C, Loquai C, Türeci Ö. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. (2017) Nature. 547:222-226.
Diken M, Vormehr M, Grunwitz C, Kreiter S, Türeci Ö, Sahin U. Discovery and subtyping of neo-epitope specific T-cell responses for cancer immunotherapy: Addressing the mutanome. In: Methods in Molecular Biology. Vol 1499. (2017) Humana Press Inc. 223-236.
Kaps L*, Nuhn L*, Aslam M, Brose A, Foerster F, Rosigkeit S, Renz P, Heck R, Kim YO, Lieberwirth I, Schuppan D, Zentel R. In Vivo Gene-Silencing in Fibrotic Liver by siRNA-Loaded Cationic Nanohydrogel Particles. (2015) Adv Healthc Mater. 4:2809-2815
*Geteilte Autorenschaft
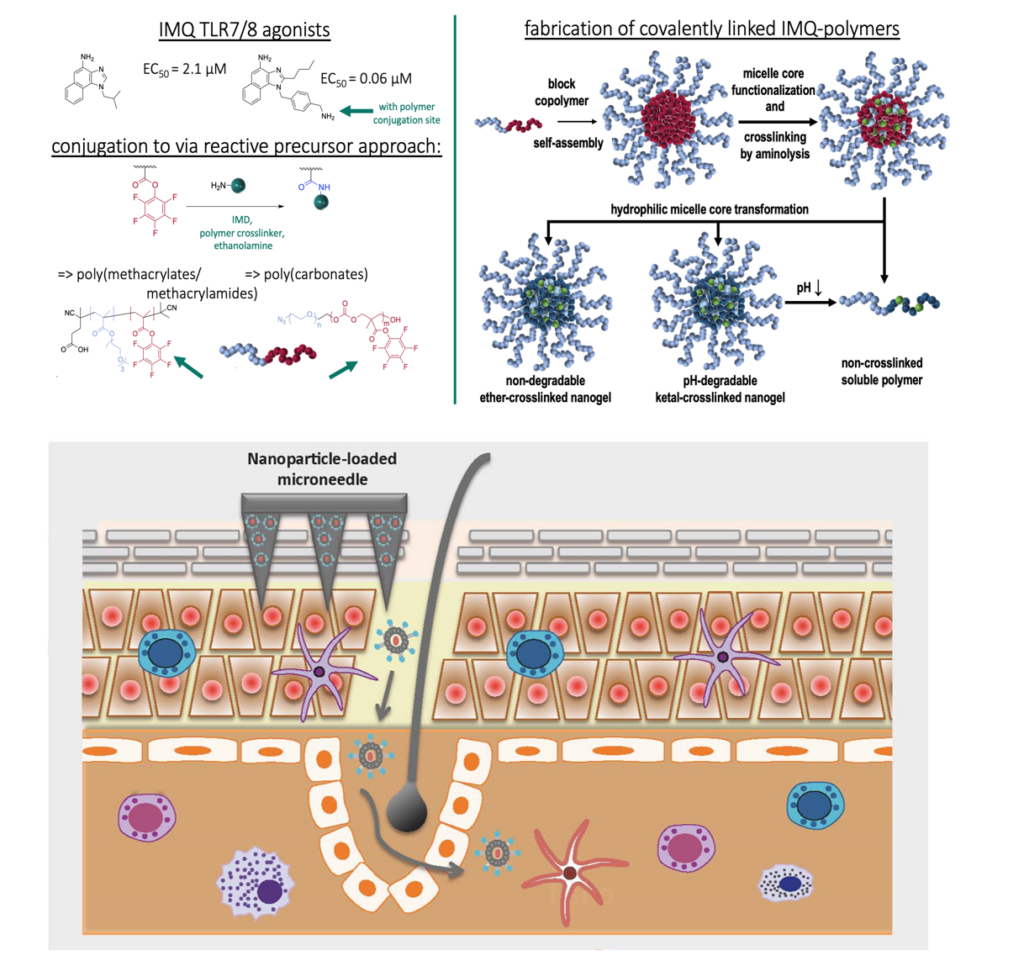
Aim of TP B18N is the pharmaceutical, preclinical and clinical development of a novel nanoparticular vaccination platform for the treatment of patients with polyoma virus associated merkel cell carcinoma (PyV-MCC). We will establish a suitable application format for our patented transcutaneous immunization method to test tolerability and efficacy in a polyoma virus induced tumor model and analyze the modes of action. Beyond this, the formal prerequisites for conduction of a clinical study in PyV-MCC patients will be installed.
P. Langguth, M. Radsak, S. Grabbe
Project relevant publications
Pielenhofer J, Sohl J, Windbergs M, Langguth P, Radsak MP. Current Progress in Particle-Based Systems for Transdermal Vaccine Delivery. (2020) Front Immunol. 11:266.
Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D, Kuhn AN, Omokoko T, Kranz LM, Diken M, Kreiter S, Haas H, Attig S, Rae R, Cuk K, Kemmer-Brück A, Breitkreuz A, Tolliver C, Caspar J, Quinkhardt J, Hebich L, Stein M, Hohberger A, Vogler I, Liebig I, Renken S, Sikorski J, Leierer M, Müller V, Mitzel-Rink H, Miederer M, Huber C, Grabbe S, Utikal J, Pinter A, Kaufmann R, Hassel JC, Loquai C, Türeci Ö. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. (2020) Nature. 585(7823):107-112.
Bialojan A, Sohl J, Rausch J, Aranda Lopez P, Denny M, Langguth P, Hartmann AK, Yagita H, Probst HC, Schild H, Radsak MP. Transcutaneous immunization with CD40 ligation boosts cytotoxic T lymphocyte mediated antitumor immunity independent of CD4 helper cells in mice. (2019) Eur. J Immunol. 49(11):2083-2094.
Teschner D, Cholaszczyńska A, Ries F, Beckert H, Theobald M, Grabbe S, Radsak MP, Bros M. (2019) CD11b regulates fungal outgrowth but not neutrophil recruitment in a mouse model of invasive pulmonary aspergillosis. (2019) Front. Immunol. 10:123.
Rausch J, Lopez PA, Bialojan A, Denny M, Langguth P, Probst HC, Schild H, Radsak MP. Combined immunotherapy: CTLA-4 blockade potentiates anti-tumor response induced by transcutaneous immunization. (2017) J. Dermatol. Sci. 87(3):300-306.
Lopez PA, Denny M, Hartmann AK, Alflen A, Probst HC, von Stebut E, Tenzer S, Schild H, Stassen M, Langguth P, Radsak MP. Transcutaneous immunization with a novel imiquimod nanoemulsion induces superior T cell responses and virus protection. (2017) J. Dermatol. Sci. 87(3):252-259.