Literature database
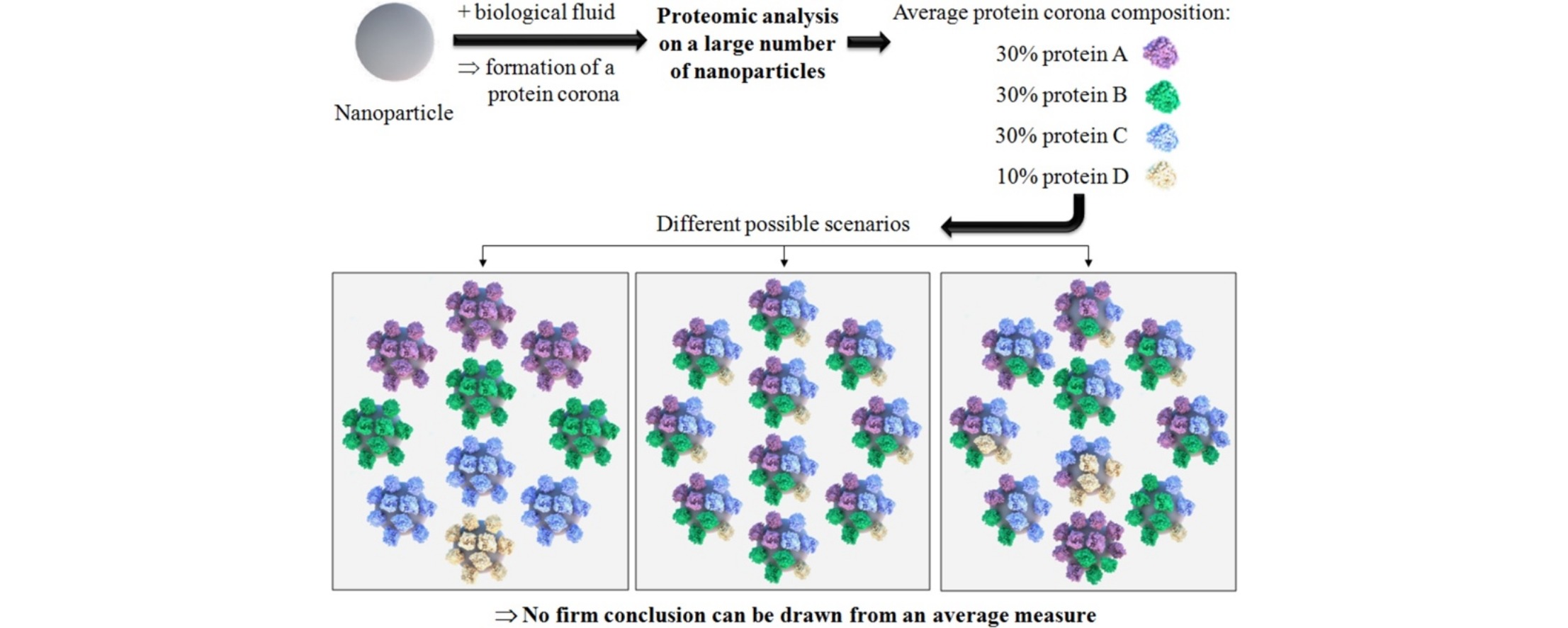
The Nanoparticle Protein Corona: The Myth of Average
Forest, V.; Pourchez, J
Nano Today 2016, 11 (6), 700–703. https://doi.org/10.1016/j.nantod.2015.10.007
Shows important limitations of averaging across biological samples especially concerning the protein corona.
Influence of Dynamic Flow Conditions on Adsorbed Plasma Protein Corona and Surface-Induced Thrombus Generation on Antifouling Brushes
Yu, K.; Andruschak, P.; Yeh, H. H.; Grecov, D.; Kizhakkedathu, J. N.
Biomaterials 2018, 166, 79–95. https://doi.org/10.1016/j.biomaterials.2018.03.009
Highlights the impact of dynamic flow conditions upon the protein corona.
The Intracellular Destiny of the Protein Corona: A Study on Its Cellular Internalization and Evolution
Bertoli, F.; Garry, D.; Monopoli, M. P.; Salvati, A.; Dawson, K. A.
ACS Nano 2016, 10 (11), 10471–10479. https://doi.org/10.1021/acsnano.6b06411
Investigates the intracellular “biological identity” of a nanoparticle defined by the protein corona.
Legal and Practical Challenges in Classifying Nanomaterials According to Regulatory Definitions
Miernicki, M.; Hofmann, T.; Eisenberger, I.; von der Kammer, F.; Praetorius, A.
Nature Publishing Group March 1, 2019, pp 208–216. https://doi.org/10.1038/s41565-019-0396-z
Shows problems of characterizing nanomaterials together with the underlying methods.
Nanoparticles Promote in Vivo Breast Cancer Cell Intravasation and Extravasation by Inducing Endothelial Leakiness
Peng, F.; Setyawati, M. I.; Tee, J. K.; Ding, X.; Wang, J.; Nga, M. E.; Ho, H. K.; Leong, D. T.
Nat. Nanotechnol. 2019, 14 (3), 279–286. https://doi.org/10.1038/s41565-018-0356-z
Shows important disadvantages of nanotherapeutics that need to be considered.
Nanomedicine and Macroscale Materials in Immuno-Oncology
Sun, Q.; Barz, M.; De Geest, B. G.; Diken, M.; Hennink, W. E.; Kiessling, F.; Lammers, T.; Shi, Y.
Chemical Society Reviews. Royal Society of Chemistry January 7, 2019, pp 351–381. https://doi.org/10.1039/c8cs00473k
Übersichtsartikel über Makromoleküle in der Zelltherapie mit allgemeiner Einführung in Drug Delivery Systeme
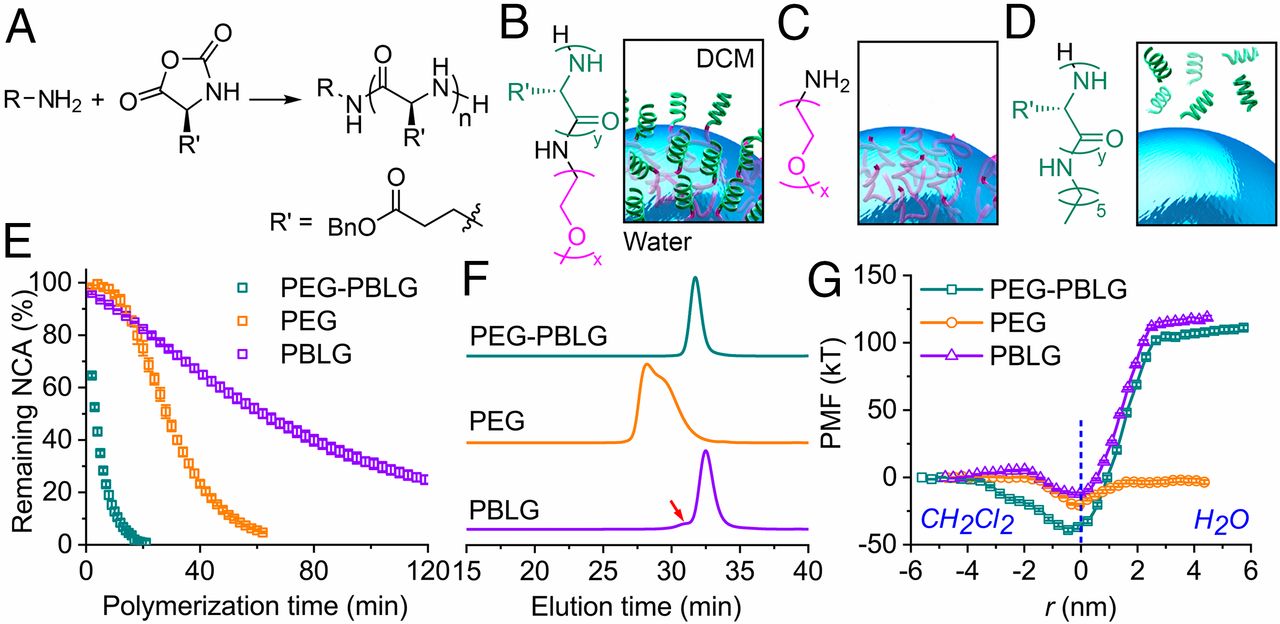
Synthesis of Polypeptides via Bioinspired Polymerization of in Situ Purified N-Carboxyanhydrides
Song, Z.; Fu, H.; Wang, J.; Hui, J.; Xue, T.; Pacheco, L. A.; Yan, H.; Baumgartner, R.; Wang, Z.; Xia, Y.; Wang, X.; Yin, L.; Chen, C.; Rodríguez-López, J.; Ferguson, A. L.; Lin, Y.; Cheng, J.
Proc. Natl. Acad. Sci. U. S. A. 2019, 166 (22), 10658–10663. https://doi.org/10.1073/pnas.1901442116
Song et al. were able to synthesize well-defined PBLG polymers with high chain lengths in a very short time even without extensive monomer purification by using a biphasic water/DCM solvent system. This approach is able to combine easy upscaling with the reduction of monomer purification and therefore, displays important progress in research of NCA and polypeptide synthesis.
Dynamics of Amphiphilic Block Copolymers in an Aqueous Solution: Direct Imaging of Micelle Formation and Nanoparticle Encapsulation
Li, C.; Tho, C. C.; Galaktionova, D.; Chen, X.; Král, P.; Mirsaidov, U.
Nanoscale 2019, 11 (5), 2299–2305. https://doi.org/10.1039/c8nr08922a
Li et al. enable a deeper look into the aggregation behavior and mechanics of amphiphilic polymers into micelles.
Evasion of the Accelerated Blood Clearance Phenomenon by Polysarcosine Coating of Liposomes
Son, K.; Ueda, M.; Taguchi, K.; Maruyama, T.; Takeoka, S.; Ito, Y.
J. Control. Release 2020, 322, 209–216. https://doi.org/10.1016/j.jconrel.2020.03.022
This paper displays the relevance of research around PEG-alternatives and the advantages of pSar as shielding material for delivery systems.
In Vivo Rendezvous of Small Nucleic Acid Drugs with Charge-Matched Block Catiomers to Target Cancers
Watanabe, S.; Hayashi, K.; Toh, K.; Kim, H. J.; Liu, X.; Chaya, H.; Fukushima, S.; Katsushima, K.; Kondo, Y.; Uchida, S.; Ogura, S.; Nomoto, T.; Takemoto, H.; Cabral, H.; Kinoh, H.; Tanaka, H. Y.; Kano, M. R.; Matsumoto, Y.; Fukuhara, H.; Uchida, S.; Nangaku, M.; Osada, K.; Nishiyama, N.; Miyata, K.; Kataoka, K.
Nat. Commun. 2019, 10 (1), 14. https://doi.org/10.1038/s41467-019-09856-w
Watanabe et al. present a siRNA delivery system that shows major differences from all others in this field. The carrier system is simply build from the siRNA and two polymers having an exact charge match with the nucleic acid molecule. Therfore, the system is really small, does not need extra stabilization and showed good transfection efficiencies even in hard-accessible tumor models.
Identification of CSPG4 as a Promising Target for Translational Combinatorial Approaches in Osteosarcoma
Riccardo, F.; Tarone, L.; Iussich, S.; Giacobino, D.; Arigoni, M.; Sammartano, F.; Morello, E.; Martano, M.; Gattino, F.; Maria, R. De; Ferrone, S.; Buracco, P.; Cavallo, F.
Ther. Adv. Med. Oncol. 2019, 11. https://doi.org/10.1177/1758835919855491
The working group was investigating the CSPG4 immune-targeting as a promising weapon for the treatment of CSPG4-positive OSA (Osteosarcoma) canine patients, to be successfully translated to a human setting
Supramolecular Nanotheranostics
Chen, X.; Zheng, G.; Cheng, J.; Yang, Y. Y.
Theranostics. Ivyspring International Publisher 2019, pp 3014–3016. https://doi.org/10.7150/thno.36788
This supramolecular nanotheranostics special issue collected a total of 17 review articles and 3 research articles broadly covering the current and emerging supramolecular nanotheranostics.
An Antibody-Drug Conjugate Targeting MUC1-Associated Carbohydrate CA6 Shows Promising Antitumor Activities
Nicolazzi, C.; Caron, A.; Tellier, A.; Trombe, M.; Pinkas, J.; Payne, G.; Carrez, C.; Guérif, S.; Maguin, M.; Baffa, R.; Fassan, M.; Adam, J.; Mangatal-Wade, L.; Blanc, V.
Mol. Cancer Ther. 2020, 19 (8), 1660–1669. https://doi.org/10.1158/1535-7163.MCT-19-0826
The humanizied antibody and antibody- derivates of SAR66658 shows high effect in different xenograft tumors
Structural Deciphering of the NG2/CSPG4 Proteoglycan Multifunctionality
Tamburini, E.; Dallatomasina, A.; Quartararo, J.; Cortelazzi, B.; Mangieri, D.; Lazzaretti, M.; Perris, R.
FASEB Journal. John Wiley and Sons Inc. March 1, 2019, pp 3112–3128. https://doi.org/10.1096/fj.201801670R
Information about the structural-functional bases for the ability of CSPG4 to widely impact on cell behavior, such as to highlight how its multivalency may be exploited to interfere with disease progression
Mouse Models for Cancer Immunotherapy Research
Olson, B.; Li, Y.; Lin, Y.; Liu, E. T.; Patnaik, A.
Cancer Discovery. American Association for Cancer Research Inc. November 1, 2018, pp 1358–1365. https://doi.org/10.1158/2159-8290.CD-18-0044
pros and cons of different cancer mouse models, and how they can be used as platforms to predict efficacy and resistance to cancer
Immunization with Mannosylated Nanovaccines and Inhibition of the Immune-Suppressing Microenvironment Sensitizes Melanoma to Immune Checkpoint Modulators
Conniot, J.; Scomparin, A.; Peres, C.; Yeini, E.; Pozzi, S.; Matos, A. I.; Kleiner, R.; Moura, L. I. F.; Zupančič, E.; Viana, A. S.; Doron, H.; Gois, P. M. P.; Erez, N.; Jung, S.; Satchi-Fainaro, R.; Florindo, H. F.
Nat. Nanotechnol. 2019, 14 (9), 891–901. https://doi.org/10.1038/s41565-019-0512-0
Mannosylated PLGA-NP were loaded with melanoma-associated peptides and TLR4 and 9 agonists (MPLA and CpG) and combined with anti-PD-1 antibody (αPD-1) for immunosuppression blockade, an anti-OX40 antibody (αOX40) for effector T-cell stimulation and ibrutinib, a myeloid-derived suppressor cell inhibitor. Synergism of the components lead to reduced tumor growth while monotherapies only showed modest results.
Peptide–TLR-7/8a Conjugate Vaccines Chemically Programmed for Nanoparticle Self-Assembly Enhance CD8 T-Cell Immunity to Tumor Antigens
Lynn, G. M.; Sedlik, C.; Baharom, F.; Zhu, Y.; Ramirez-Valdez, R. A.; Coble, V. L.; Tobin, K.; Nichols, S. R.; Itzkowitz, Y.; Zaidi, N.; Gammon, J. M.; Blobel, N. J.; Denizeau, J.; de la Rochere, P.; Francica, B. J.; Decker, B.; Maciejewski, M.; Cheung, J.; Yamane, H.; Smelkinson, M. G.; Francica, J. R.; Laga, R.; Bernstock, J. D.; Seymour, L. W.; Drake, C. G.; Jewell, C. M.; Lantz, O.; Piaggio, E.; Ishizuka, A. S.; Seder, R. A.
Nat. Biotechnol. 2020, 38 (3), 320–332. https://doi.org/10.1038/s41587-019-0390-x
A platform that self-assembles into NP based on conjugates of TLR7/8 agonists and synthetic long peptides (of different neoepitopes) increases uptake and activation of APCs. The vaccine platform leads to reduced tumor growth. I.v and s.c. vaccinations are compared.
In Vivo Characterization of the Physicochemical Properties of Polymer-Linked TLR Agonists That Enhance Vaccine Immunogenicity
Lynn, G. M.; Laga, R.; Darrah, P. A.; Ishizuka, A. S.; Balaci, A. J.; Dulcey, A. E.; Pechar, M.; Pola, R.; Gerner, M. Y.; Yamamoto, A.; Buechler, C. R.; Quinn, K. M.; Smelkinson, M. G.; Vanek, O.; Cawood, R.; Hills, T.; Vasalatiy, O.; Kastenmüller, K.; Francica, J. R.; Stutts, L.; Tom, J. K.; Ryu, K. A.; Esser-Kahn, A. P.; Etrych, T.; Fisher, K. D.; Seymour, L. W.; Seder, R. A.
Nat. Biotechnol. 2015, 33 (11), 1201–1210. https://doi.org/10.1038/nbt.3371
A TLR7/8 agonist was linked to a polymer and it was evaluated how physicochemical properties (density of agonist, particle formation, ..) influences immune activation in vivo. It was found that particle formation is critical for a prolonged immune activation in the draining lymph nodes and uptake into APCs.
Mucosal Immunization with a PH-Responsive Nanoparticle Vaccine Induces Protective CD8+ Lung-Resident Memory T Cells
Knight, F. C.; Gilchuk, P.; Kumar, A.; Becker, K. W.; Sevimli, S.; Jacobson, M. E.; Suryadevara, N.; Wang-Bishop, L.; Boyd, K. L.; Crowe, J. E.; Joyce, S.; Wilson, J. T.
ACS Nano 2019, 13 (10), 10939–10960. https://doi.org/10.1021/acsnano.9b00326
The capacity to generate antigen-specific CD8+ T cells of dual-loaded NP (with OVA and CpG) from pH-responsiven diblock copolymers is tested after intranasal application. Application of various combinations of OVA/NP/CpG shows that co-delivery of adjuvant and antigen is crucial.
Antigens Reversibly Conjugated to a Polymeric Glyco-Adjuvant Induce Protective Humoral and Cellular Immunity
Wilson, D. S.; Hirosue, S.; Raczy, M. M.; Bonilla-Ramirez, L.; Jeanbart, L.; Wang, R.; Kwissa, M.; Franetich, J. F.; Broggi, M. A. S.; Diaceri, G.; Quaglia-Thermes, X.; Mazier, D.; Swartz, M. A.; Hubbell, J. A.
Nat. Mater. 2019, 18 (2), 175–185. https://doi.org/10.1038/s41563-018-0256-5
A RAFT-based system consisting of TLR7 agonist-, mannose- und HPMA-monomers is combined with a self-immolative linker to later on release a native protein antigen (OVA). The study shows that the covalent conjugation of OVA, TLR7 agonist and mannose generates greater humoral and cellular immunity than OVA conjugated to polymers lacking either mannose targeting or TLR7 ligand.
Potent and Prolonged Innate Immune Activation by Enzyme-Responsive Imidazoquinoline TLR7/8 Agonist Prodrug Vesicles
Wang, B.; Van Herck, S.; Chen, Y.; Bai, X.; Zhong, Z.; Deswarte, K.; Lambrecht, B. N.; Sanders, N. N.; Lienenklaus, S.; Scheeren, H. W.; David, S. A.; Kiessling, F.; Lammers, T.; De Geest, B. G.; Shi, Y.
J. Am. Chem. Soc. 2020, 142 (28), 12133–12139. https://doi.org/10.1021/jacs.0c01928
Die Autoren berichten über die Entwicklung eines als mizellar selbst-assemblierendes Pro-Drug designten TLR7/8 Agonisten (IMDQ), der nach Zellaufnahme durch Nutzung eines Enzymlabilen Linkers unter Zurückbildung der Überstruktur freigesetzt wird.
Controlled Lengthwise Assembly of Helical Peptide Nanofibers to Modulate CD8 + T‐Cell Responses
Fries, C. N.; Wu, Y.; Kelly, S. H.; Wolf, M.; Votaw, N. L.; Zauscher, S.; Collier, J. H.
Adv. Mater. 2020, 32 (39), 2003310. https://doi.org/10.1002/adma.202003310
Die Publikation veranschaulicht das Konzept des Cappings supramolekularen Peptid-Nanofasern um den Einfluss der Länge auf ihre Immunogenität, hier erfasst durch die Stärke der induzierten CD8-T-Zell Antwort, zu untersuchen und zeigt zugleich ein Design zur Beeinflussung der Länge.
Endosomolytic Polymersomes Increase the Activity of Cyclic Dinucleotide STING Agonists to Enhance Cancer Immunotherapy
Shae, D.; Becker, K. W.; Christov, P.; Yun, D. S.; Lytton-Jean, A. K. R.; Sevimli, S.; Ascano, M.; Kelley, M.; Johnson, D. B.; Balko, J. M.; Wilson, J. T.
Nat. Nanotechnol. 2019, 14 (3), 269–278. https://doi.org/10.1038/s41565-018-0342-5
Die Autoren beschreiben STING-aktivierende Nanopartikel (STING-NPs)-rational entworfene Polymere für eine verbesserte zytosolische Freisetzung des endogenen CDN-Liganden von STING, 2′3′ cGAMP.
Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release
Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O. C.
Chemical Reviews. American Chemical Society 2016, pp 2602–2663. https://doi.org/10.1021/acs.chemrev.5b00346
Der Übersichtsartikel resümiert die Verwendung bioabbaubarer Polymere zur kontrollierten Freisetzung von Medikamenten und erklärt die diesbezüglich relevanten physikalischen, chemischen und biologischen Parameter.
Efficient Innate Immune Killing of Cancer Cells Triggered by Cell-Surface Anchoring of Multivalent Antibody-Recruiting Polymers
Uvyn, A.; De Coen, R.; Gruijs, M.; Tuk, C. W.; De Vrieze, J.; van Egmond, M.; De Geest, B. G.
Angew. Chemie – Int. Ed. 2019, 58 (37), 12988–12993. https://doi.org/10.1002/anie.201905093
Antikörper rekrutierende Polymere (ARPs), die aus einem zellbindenden Motiv bestehen, das mit einem Polymer verbunden ist, das mehrere niedermolekulare Antikörper‐Bindungsmotive entlang seines Rückgrats enthält, wurden verwendet, um Krebszellen zur Zerstörung durch das angeborene Immunsystem zu markieren.
Dendritic Cells Tip the Balance towards Induction of Regulatory T Cells upon Priming in Experimental Autoimmune Encephalomyelitis
Paterka, M.; Voss, J. O.; Werr, J.; Reuter, E.; Franck, S.; Leuenberger, T.; Herz, J.; Radbruch, H.; Bopp, T.; Siffrin, V.; Zipp, F.
J. Autoimmun. 2017, 76, 108–114. https://doi.org/10.1016/j.jaut.2016.09.008
DCs facilitate iTreg induction à Create a milieu with high levels of IL-2; Absence of DCs = B220+ B cells take over priming of Th17 cells
Murine Myeloproliferative Disorder as a Consequence of Impaired Collaboration between Dendritic Cells and CD4 T Cells
Humblet-Baron, S.; Barber, J. S.; Roca, C. P.; Lenaerts, A.; Koni, P. A.; Liston, A.
Blood 2019, 133 (4), 319–330. https://doi.org/10.1182/blood-2018-05-850321
DC functional impairment drives myeloproliferative disease; Myeloproliferative disorder development relies on the presence of CD4 T cells
Extracellular Vesicle–Mediated Transfer of Constitutively Active MyD88 L265P Engages MyD88 Wt and Activates Signaling
Manček-Keber, M.; Lainšček, D.; Benčina, M.; Chen, J. G.; Romih, R.; Hunter, Z. R.; Treon, S. P.; Jerala, R.
Blood 2018, 131 (15), 1720–1729. https://doi.org/10.1182/blood-2017-09-805499
Results establish the mechanism of transmission of signaling complexes via extracellular vesicles to propagate inflammation as a new mechanism of intercellular communication
Selective Organ Targeting (SORT) Nanoparticles for Tissue-Specific MRNA Delivery and CRISPR–Cas Gene Editing
Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L. T.; Dilliard, S. A.; Siegwart, D. J.
Nat. Nanotechnol. 2020, 15 (4), 313–320. https://doi.org/10.1038/s41565-020-0669-6
Einführung der SORT (Selective organ targeting) Strategie zur Synthese von Nanopartikeln für den gezielten Transport von RNA zu spezifischen Organen
The Size and Composition of Polymeric Nanocapsules Dictate Their Interaction with Macrophages and Biodistribution in Zebrafish
Crecente-Campo, J.; Guerra-Varela, J.; Peleteiro, M.; Gutiérrez-Lovera, C.; Fernández-Mariño, I.; Diéguez-Docampo, A.; González-Fernández, Á.; Sánchez, L.; Alonso, M. J.
J. Control. Release 2019, 308, 98–108. https://doi.org/10.1016/j.jconrel.2019.07.011
In vivo und in vitro Untersuchung des Einflusses von Partikelgröße und Zusammensetzung der Schale auf die Interaktionen mit Makrophagen
Crosslinking: An Avenue to Develop Stable Amorphous Solid Dispersion with High Drug Loading and Tailored Physical Stability
Sahoo, A.; Kumar, N. S. K.; Suryanarayanan, R.
J. Control. Release 2019, 311–312, 212–224. https://doi.org/10.1016/j.jconrel.2019.09.007
Einfluss des Vernetzungsgrades auf physikalische Stabilität und Wirkstoffeinschluss am Beispiel von amorphen Dispersionen
Complement Activation Turnover on Surfaces of Nanoparticles
Moghimi, S. M.; Simberg, D.
Nano Today 2017, 15, 8–10. https://doi.org/10.1016/j.nantod.2017.03.001
Betrachtung des Designs und der Pharmakokinetik von Nanopartikeln hinsichtlich der verwendeten Materialien und auftretender Wechselwirkungen mit dem Komplementsystem
Stimulus-Responsive Sequential Release Systems for Drug and Gene Delivery
Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N. T.; Valibeik, A.; Karimi, M.; Hamblin, M. R.
Nano Today. Elsevier B.V. October 1, 2020. https://doi.org/10.1016/j.nantod.2020.100914
Review über die verschiedenen Formen der sequentiellen Wirkstofffreisetzung und deren Responsivität gegenüber diversen Stimuli.
Liposome-Encapsulated Plasmid DNA of Telomerase-Specific Oncolytic Adenovirus with Stealth Effect on the Immune System
Aoyama, K.; Kuroda, S.; Morihiro, T.; Kanaya, N.; Kubota, T.; Kakiuchi, Y.; Kikuchi, S.; Nishizaki, M.; Kagawa, S.; Tazawa, H.; Fujiwara, T.
Sci. Rep. 2017, 7 (1), 1–10. https://doi.org/10.1038/s41598-017-14717-x
Eine Studie, die den Einfluss der Partikelgröße auf endosomales Trafficking untersucht
Analysis of Mesenchymal Stem Cell Proteomes in Situ in the Ischemic Heart
Han, D.; Yang, J.; Zhang, E.; Liu, Y.; Boriboun, C.; Qiao, A.; Yu, Y.; Sun, J.; Xu, S.; Yang, L.; Yan, W.; Luo, B.; Lu, D.; Zhang, C.; Jie, C.; Mobley, J.; Zhang, J.; Qin, G.
Theranostics 2020, 10 (24), 11324–11338. https://doi.org/10.7150/thno.47893
Ein themenverwantes Paper zu Q2 und der Erschließung des intrazellulären Traffickings von Nanopartikeln mittels Proteomics
Concepts of Nanoparticle Cellular Uptake, Intracellular Trafficking, and Kinetics in Nanomedicine
Donahue, N. D.; Acar, H.; Wilhelm, S.
Advanced Drug Delivery Reviews. Elsevier B.V. March 15, 2019, pp 68–96. https://doi.org/10.1016/j.addr.2019.04.008
Ein gutes Review Paper zur Aufnahme und dem intrazellulären Trafficking von Nanopartikeln, auch zu endosomalen Transportwegen
Zr-Immuno-PET: Toward a Noninvasive Clinical Tool to Measure Target Engagement of Therapeutic Antibodies In Vivo
Jauw, Y. W. S.; O’Donoghue, J. A.; Zijlstra, J. M.; Hoekstra, O. S.; Menke-van der Houven van Oordt, C. W.; Morschhauser, F.; Carrasquillo, J. A.; Zweegman, S.; Pandit-Taskar, N.; Lammertsma, A. A.; van Dongen, G. A. M. S.; Boellaard, R.; Weber, W. A.; Huisman, M. C.
J. Nucl. Med. 2019, 60 (12), 1825–1832. https://doi.org/10.2967/jnumed.118.224568
In dieser Publikation stellen Jauw et al. die Auswertung einiger verschiedener klinischer Studien 89Zr-markierter Antikörperkonjugate mit Schwerpunkt auf die reversible und irreversible unspezifische Aufnahme in gesundem Gewebe als kritischen Parameter zur Quantifizierung der Wirksamkeit bei Tumoren vor.
Photochemical Conjugation and One-Pot Radiolabelling of Antibodies for Immuno-PET
Patra, M.; Eichenberger, L. S.; Fischer, G.; Holland, J. P.
Angew. Chemie – Int. Ed. 2019, 58 (7), 1928–1933. https://doi.org/10.1002/anie.201813287
In dieser Publikation stellen Patra et al. eine innovative photochemische One-Pot Synthese zur Darstellung radiomarkierter Antikörperkonjugate für die Immuno-PET vor, die eine schnelle chemoselektive Alternative zu konventionellen mehrstufigen Methoden darstellt.
Biokinetics of Radiolabeled Monoclonal Antibody BC8: Differences in Biodistribution and Dosimetry Among Hematologic Malignancies
Matesan, M.; Fisher, D. R.; Wong, R.; Gopal, A. K.; Green, D. J.; Sandmaier, B. M.; Bensinger, W.; Pagel, J. M.; Orozco, J.; Press, O. W.; Cassaday, R. D.; Hutchinson, E.; Wanner, M.; Pal, S.; Thostenson, C.; Rajendran, J. G.
J. Nucl. Med. 2020, 61 (9), 1300–1306. https://doi.org/10.2967/jnumed.119.234443
Diese Publikation beschreibt die Verwendung eines 111In-markierten, CD45-spezifischen Antikörpers bei Patienten mit verschiedenen hämatologischen Krebserkrankungen zur SPECT-Darstellung der unterschiedlichen Biodistribution und Pharmakokinetik.
ImmunoPET with Anti-Mesothelin Antibody in Patients with Pancreatic and Ovarian Cancer before Anti-Mesothelin Antibody–Drug Conjugate Treatment
Lamberts, L. E.; Menke-van der Houven van Oordt, C. W.; ter Weele, E. J.; Bensch, F.; Smeenk, M. M.; Voortman, J.; Hoekstra, O. S.; Williams, S. P.; Fine, B. M.; Maslyar, D.; de Jong, J. R.; Gietema, J. A.; Schröder, C. P.; Bongaerts, A. H. H.; Lub-de Hooge, M. N.; Verheul, H. M. W.; Sanabria Bohorquez, S. M.; Glaudemans, A. W. J. M.; de Vries, E. G. E.
Clin. Cancer Res. 2016, 22 (7), 1642–1652. https://doi.org/10.1158/1078-0432.CCR-15-1272
Diese Publikation beschreibt die Verwendung eines 89Zr-markierten, Mesothelin-spezifischen Antikörpers zur Evaluierung des Tumoranreicherung, der Ganzkörperverteilung und des Therapieerfolgs bei Patienten mit Ovarial- und Pankreaskarzinomen und stellt somit ein aktuelles klinisches Anwendungsbeispiel der Immuno-PET dar.
Biological Identity of Nanoparticles In Vivo: Clinical Implications of the Protein Corona
Caracciolo, G.; Farokhzad, O. C.; Mahmoudi, M.
Trends in Biotechnology. Elsevier Ltd March 1, 2017, pp 257–264. https://doi.org/10.1016/j.tibtech.2016.08.011
A review of recent advances in the understanding of the in vivo protein corona and development of quantitative models to predict biological interactions.
A Minimal Physiologically Based Pharmacokinetic Model That Predicts Anti-PEG IgG-Mediated Clearance of PEGylated Drugs in Human and Mouse
McSweeney, M. D.; Wessler, T.; Price, L. S. L.; Ciociola, E. C.; Herity, L. B.; Piscitelli, J. A.; Zamboni, W. C.; Forest, M. G.; Cao, Y.; Lai, S. K.
J. Control. Release 2018, 284, 171–178. https://doi.org/10.1016/j.jconrel.2018.06.002
They developed a model (mPBPK) to capture the impact of anti-PEG antibodies on the pharmacokinetics of PEGylated entities in humans and mice, thus providing a valuable tool for a personalized medicine approach by selecting doses of PEGylated therapeutics for sensitized individuals.
Measurement of Pre-Existing IgG and IgM Antibodies against Polyethylene Glycol in Healthy Individuals
Chen, B. M.; Su, Y. C.; Chang, C. J.; Burnouf, P. A.; Chuang, K. H.; Chen, C. H.; Cheng, T. L.; Chen, Y. T.; Wu, J. Y.; Roffler, S. R.
Anal. Chem. 2016, 88 (21), 10661–10666. https://doi.org/10.1021/acs.analchem.6b03109
This paper describes a high prevalence of per-existing anti-PEG antibodies (IgG and IgM) in healthy Han Chinese donors measured via a newly developed ELISA.
Corona Composition Can Affect the Mechanisms Cells Use to Internalize Nanoparticles
Francia, V.; Yang, K.; Deville, S.; Reker-Smit, C.; Nelissen, I.; Salvati, A.
ACS Nano 2019, 13 (10), 11107–11121. https://doi.org/10.1021/acsnano.9b03824
This paper describes that the same nanoparticles can enter cells via different mechanisms when coated by coronas of different composition only following dispersion in different protein contents.
Anti-Polyethylene Glycol Antibodies Alter the Protein Corona Deposited on Nanoparticles and the Physiological Pathways Regulating Their Fate in Vivo
Grenier, P.; Viana, I. M. de O.; Lima, E. M.; Bertrand, N.
J. Control. Release 2018, 287, 121–131. https://doi.org/10.1016/j.jconrel.2018.08.022
The presence of anti-PEG IgM, after the injection of PEG-PLGA nanoparticles, induces a neutralizing effect on the circulation of subsequent doses of nanoparticles but not free mPEG and PEG-BSA.
Cloaking Nanoparticles with Protein Corona Shield for Targeted Drug Delivery
Oh, J. Y.; Kim, H. S.; Palanikumar, L.; Go, E. M.; Jana, B.; Park, S. A.; Kim, H. Y.; Kim, K.; Seo, J. K.; Kwak, S. K.; Kim, C.; Kang, S.; Ryu, J. H.
Nat. Commun. 2018, 9 (1), 1–9. https://doi.org/10.1038/s41467-018-06979-4
Demonstrating the issues of nanocarriers in a biological environment this article presents the design and advantages of protein precoated nanoparticle as efficient drug delivery system.
Feedback-Induced Temporal Control of “Breathing” Polymersomes to Create Self-Adaptive Nanoreactors
Che, H.; Cao, S.; Van Hest, J. C. M.
J. Am. Chem. Soc. 2018, 140 (16), 5356–5359. https://doi.org/10.1021/jacs.8b02387
This article presents the development of a protein loaded, pH-sensitive polymersome as potential self-regulated nanocarrier.
Feedback-Induced Temporal Control of “Breathing” Polymersomes to Create Self-Adaptive Nanoreactors
Che, H.; Cao, S.; Van Hest, J. C. M.
J. Am. Chem. Soc. 2018, 140 (16), 5356–5359. https://doi.org/10.1021/jacs.8b02387
This article presents the development of a protein loaded, pH-sensitive polymersome as potential self-regulated nanocarrier.
Tumor-Acidity-Cleavable Maleic Acid Amide (TACMAA): A Powerful Tool for Designing Smart Nanoparticles to Overcome Delivery Barriers in Cancer Nanomedicine
Du, J. Z.; Li, H. J.; Wang, J.
Acc. Chem. Res. 2018, 51 (11), 2848–2856. https://doi.org/10.1021/acs.accounts.8b00195
Summarizing the biological delivery barriers in the path of a nanomedicine this article illustrates the development and design of TACMAA-based nanocarriers as potential drug delivery system.
Induction of Anti-Cancer T Cell Immunity by in Situ Vaccination Using Systemically Administered Nanomedicines
Lynn, G. M.; Laga, R.; Jewell, C. M.
Cancer Letters. Elsevier Ireland Ltd September 10, 2019, pp 192–203. https://doi.org/10.1016/j.canlet.2019.114427
This article provides detailed information on the design of micellar-and star polymer-based nanoparticles for drug delivery, focusing the usage of nanomedicine for anti-cancer T cell immunity by vaccination.
Bioinspired Hybrid Protein Oxygen Nanocarrier Amplified Photodynamic Therapy for Eliciting Anti-Tumor Immunity and Abscopal Effect
Chen, Z.; Liu, L.; Liang, R.; Luo, Z.; He, H.; Wu, Z.; Tian, H.; Zheng, M.; Ma, Y.; Cai, L.
ACS Nano 2018, 12 (8), 8633–8645. https://doi.org/10.1021/acsnano.8b04371
In this study, human serumalbumin was hybridized with hemoglobin by intermolecular disulfide bonds to develop a hybrid protein oxygen nanocarrier with chlorine e6 encapsulated (C@HPOC) for oxygen self-sufficient photodynamic therapy (PDT), which relieved tumor hypoxia and enhanced infiltration of CD8+ T cells in tumors to destroy primary tumors and effectively supress metastasis by evoking systemic anti-tumor immunity.
Protein-Based Nanoparticles in Cancer Vaccine Development
Neek, M.; Kim, T. Il; Wang, S. W.
Nanomedicine: Nanotechnology, Biology, and Medicine. Elsevier Inc. January 1, 2019, pp 164–174. https://doi.org/10.1016/j.nano.2018.09.004
A review stating the development in peptide and protein-based nanoparticles as cancer vaccines.
Electrochemically Promoted Tyrosine-Click-Chemistry for Protein Labeling
Alvarez-Dorta, D.; Thobie-Gautier, C.; Croyal, M.; Bouzelha, M.; Mével, M.; Deniaud, D.; Boujtita, M.; Gouin, S. G.
J. Am. Chem. Soc. 2018, 140 (49), 17120–17126. https://doi.org/10.1021/jacs.8b09372
The new bio-orthogonal ligation method trough electrochemically promoted tyrosine-click enables mild and chemoselective modification of peptides and proteins with labeled urazoles.
Mapping and Identification of Soft Corona Proteins at Nanoparticles and Their Impact on Cellular Association
Mohammad-Beigi, H.; Hayashi, Y.; Zeuthen, C. M.; Eskandari, H.; Scavenius, C.; Juul-Madsen, K.; Vorup-Jensen, T.; Enghild, J. J.; Sutherland, D. S.
Nat. Commun. 2020, 11 (1), 1–16. https://doi.org/10.1038/s41467-020-18237-7
An in situ SPAAC click reaction between azide-modified hard corona proteins on nanoparticles and DBCO-activated soft corona proteins allows trapping of transiently binding soft corona proteins on nanoparticle surface to study the effect of the dynamic nature of soft corona proteins on cell association.
Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination
Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z.
ACS Nano 2018, 12 (6), 5121–5129. https://doi.org/10.1021/acsnano.7b09041
The developed nanovaccine formulation, consisted of encapsulated adjuvant Imiquimod into Poly(D,L-lactide-co-glycolide) nanoparticles coated with melanoma cell membranes as tumor-specific antigen and modified with mannose moieties for specific uptake to DCs, induces DC maturation and delays tumor growth.
Reprogramming Tumor-Associated Macrophages by Nanoparticle-Based Reactive Oxygen Species Photogeneration
Shi, C.; Liu, T.; Guo, Z.; Zhuang, R.; Zhang, X.; Chen, X.
Nano Lett. 2018, 18 (11), 7330–7342. https://doi.org/10.1021/acs.nanolett.8b03568
This strategy could effectively eradicate tumors, inhibit metastasis, and further prevent their recurrence, which holds tremendous promise to realize potent cancer immunotherapy.
Influence of Core Cross-Linking and Shell Composition of Polymeric Micelles on Immune Response and Their Interaction with Human Monocytes
Gardey, E.; Sobotta, F. H.; Hoeppener, S.; Bruns, T.; Stallmach, A.; Brendel, J. C.
Biomacromolecules 2020, 21 (4), 1393–1406. https://doi.org/10.1021/acs.biomac.9b01656
This study emphasizes the importance of the micellar stability on the interaction with the immune system, which is the key for any stealth properties in vivo.
Antibody Fragment-Conjugated Polymeric Micelles Incorporating Platinum Drugs for Targeted Therapy of Pancreatic Cancer
Ahn, J.; Miura, Y.; Yamada, N.; Chida, T.; Liu, X.; Kim, A.; Sato, R.; Tsumura, R.; Koga, Y.; Yasunaga, M.; Nishiyama, N.; Matsumura, Y.; Cabral, H.; Kataoka, K.
Biomaterials 2015, 39, 23–30. https://doi.org/10.1016/j.biomaterials.2014.10.069
These results indicate the potential of Fab‘-installed polymeric micelles for efficient drug delivery to solid tumors.
Immunogenicity of Polyethylene Glycol Based Nanomedicines: Mechanisms, Clinical Implications and Systematic Approach
d’Avanzo, N.; Celia, C.; Barone, A.; Carafa, M.; Di Marzio, L.; Santos, H. A.; Fresta, M.
Adv. Ther. 2020, 3 (3), 1900170. https://doi.org/10.1002/adtp.201900170
PEGlation is considered as Golden Standard for providing stealth properties of nanomedicines. This review summarizes the mechanism based on PEG immunogenicity and describes alternative strategies to avoid unexpected immunogenic responses.
Minimum Information Reporting in Bio–Nano Experimental Literature
Faria, M.; Björnmalm, M.; Thurecht, K. J.; Kent, S. J.; Parton, R. G.; Kavallaris, M.; Johnston, A. P. R.; Gooding, J. J.; Corrie, S. R.; Boyd, B. J.; Thordarson, P.; Whittaker, A. K.; Stevens, M. M.; Prestidge, C. A.; Porter, C. J. H.; Parak, W. J.; Davis, T. P.; Crampin, E. J.; Caruso, F.
Nat. Nanotechnol. 2018, 13 (9), 777–785. https://doi.org/10.1038/s41565-018-0246-4
Minimum information reporting in bio-nano experimental literature. 13 (9), 777-785. Nature nanotechnology 2018 31.538 Studying the interactions between nanoengineered materials and biological systems plays a vital role in the development of biological applications of nanotechnology and the improvement of our fundamental understanding of the bio-nano interface. This study suggest a ‚minimum information standard‘ for experimental literature investigating bio-nano interactions. With the intention to improve reproducibility, increase quantitative comparisons of bio-nano materials, and facilitate meta analyses and in silico modelling.
Use of Polymeric Nanoparticle Platform Targeting the Liver To Induce Treg-Mediated Antigen-Specific Immune Tolerance in a Pulmonary Allergen Sensitization Model
Liu, Q.; Wang, X.; Liu, X.; Kumar, S.; Gochman, G.; Ji, Y.; Liao, Y. P.; Chang, C. H.; Situ, W.; Lu, J.; Jiang, J.; Mei, K. C.; Meng, H.; Xia, T.; Nel, A. E.
ACS Nano 2019, 13 (4), 4778–4794. https://doi.org/10.1021/acsnano.9b01444
Nanoparticles can be used to accomplish antigen-specific immune tolerance in allergic and autoimmune disease. The available options for custom-designing tolerogenic NPs include the use of nanocarriers that introduce antigens into natural tolerogenic environments, such as the liver, where antigen presentation promotes tolerance to self- or foreign antigens. The significance of the findings in this study is, that liver and LSEC targeting PLGA NPs could be used for therapy of allergic airway disease, in addition to the potential of using their tolerogenic effects for other disease applications.
CSF1R Regulates the Dendritic Cell Pool Size in Adult Mice via Embryo-Derived Tissue-Resident Macrophages
Percin, G. I.; Eitler, J.; Kranz, A.; Fu, J.; Pollard, J. W.; Naumann, R.; Waskow, C.
Nat. Commun. 2018, 9 (1), 1–12. https://doi.org/10.1038/s41467-018-07685-x
This study showed that the differentiation of DC and their regeneration relies on ontogenetically distinct spleen macrophages, thereby providing a novel regulatory principle that may also be important for the differentiation of other hematopoietic cell types.
Coordinative Binding of Polymers to Metal-Organic Framework Nanoparticles for Control of Interactions at the Biointerface
Zimpel, A.; Al Danaf, N.; Steinborn, B.; Kuhn, J.; Höhn, M.; Bauer, T.; Hirschle, P.; Schrimpf, W.; Engelke, H.; Wagner, E.; Barz, M.; Lamb, D. C.; Lächelt, U.; Wuttke, S.
ACS Nano 2019, 13 (4), 3884–3895. https://doi.org/10.1021/acsnano.8b06287
Metal-organic framework nanoparticles (MOF NPs) are of growing interest in diagnostic and therapeutic applications, and due to their hybrid nature, they display enhanced properties compared to more established nanomaterials. In this way, the physicochemical properties of MOF NPs could be tuned, which allows for control over their behavior in biological systems.
Ligand Density on Nanoparticles: A Parameter with Critical Impact on Nanomedicine
Alkilany, A. M.; Zhu, L.; Weller, H.; Mews, A.; Parak, W. J.; Barz, M.; Feliu, N.
Advanced Drug Delivery Reviews. Elsevier B.V. March 15, 2019, pp 22–36. https://doi.org/10.1016/j.addr.2019.05.010
In this review is about how many ligands per nanoparticle are best for the most efficient delivery? These results help to understand which ligand densities should be attempted for better targeting.
Immunostimulatory Nanomedicines Synergize with Checkpoint Blockade Immunotherapy to Eradicate Colorectal Tumors
Duan, X.; Chan, C.; Han, W.; Guo, N.; Weichselbaum, R. R.; Lin, W.
Nat. Commun. 2019, 10 (1), 1–15. https://doi.org/10.1038/s41467-019-09221-x
Immunstimulierende, chemotherapeutische Nanopartikel-Kombination, langanhaltende Anti-Tumor Immunität (biodegradable, promises to enter clinical testing as immunotherapy against colorectal cancer).
Ultrasmall Targeted Nanoparticles with Engineered Antibody Fragments for Imaging Detection of HER2-Overexpressing Breast Cancer
Chen, F.; Ma, K.; Madajewski, B.; Zhuang, L.; Zhang, L.; Rickert, K.; Marelli, M.; Yoo, B.; Turker, M. Z.; Overholtzer, M.; Quinn, T. P.; Gonen, M.; Zanzonico, P.; Tuesca, A.; Bowen, M. A.; Norton, L.; Subramony, J. A.; Wiesner, U.; Bradbury, M. S.
Nat. Commun. 2018, 9 (1), 1–11. https://doi.org/10.1038/s41467-018-06271-5
Reduzierte Nanopartikel-Aufnahme in Leber und anderen Organen. Ultrakleine Nanopartikel mit Antikörper-Derivaten funktuinalisiert als drug delivery System (favorable particle biodistribution profile, not exceeding the threshold for renal filtration as a combined vehicle).
T Cell-Targeting Nanoparticles Focus Delivery of Immunotherapy to Improve Antitumor Immunity
Schmid, D.; Park, C. G.; Hartl, C. A.; Subedi, N.; Cartwright, A. N.; Puerto, R. B.; Zheng, Y.; Maiarana, J.; Freeman, G. J.; Wucherpfennig, K. W.; Irvine, D. J.; Goldberg, M. S.
Nat. Commun. 2017, 8 (1), 1–12. https://doi.org/10.1038/s41467-017-01830-8
Medikament-beladene, Antikörper-modifizierte Nanopartikel die CD8+ T Zellen in vitro als auch in vivo binden (TGFß inhibitor signaling to PD-1-expressing cells, antibody fragmentation, TLR7/8 agonist)
Pointing in the Right Direction: Controlling the Orientation of Proteins on Nanoparticles Improves Targeting Efficiency
Yong, K. W.; Yuen, D.; Chen, M. Z.; Porter, C. J. H.; Johnston, A. P. R.
Nano Lett. 2019, 19 (3), 1827–1831. https://doi.org/10.1021/acs.nanolett.8b04916
Eine orientierte und seiten-spezifische Anbringung eines Nanobodies auf die Nanopartikel-Oberfläche (targeting, peptides and proteins, monomers, fluorescence, nanoparticles)
Modified MRNA Vaccines Protect against Zika Virus Infection
Richner, J. M.; Himansu, S.; Dowd, K. A.; Butler, S. L.; Salazar, V.; Fox, J. M.; Julander, J. G.; Tang, W. W.; Shresta, S.; Pierson, T. C.; Ciaramella, G.; Diamond, M. S.
Cell 2017, 168 (6), 1114-1125.e10. https://doi.org/10.1016/j.cell.2017.02.017
Erste in-human Studie mit therapeutischen mRNA LNPs.
The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs
Akinc, A.; Maier, M. A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M. J.; Madden, T. D.; Mui, B. L.; Semple, S. C.; Tam, Y. K.; Ciufolini, M.; Witzigmann, D.; Kulkarni, J. A.; van der Meel, R.; Cullis, P. R.
Nature Nanotechnology. Nature Research December 1, 2019, pp 1084–1087. https://doi.org/10.1038/s41565-019-0591-y
Geschichte des ersten zugelassenen LNP-Therapeutikums (Onpattro®)
DLS and Zeta Potential - What They Are and What They Are Not?
Bhattacharjee, S.
Journal of Controlled Release. Elsevier B.V. August 10, 2016, pp 337–351. https://doi.org/10.1016/j.jconrel.2016.06.017
DLS and Zeta Potential – What They Are and What They Are Not?
Identifying Key Barriers in Cationic Polymer Gene Delivery to Human T Cells
Olden, B. R.; Cheng, E.; Cheng, Y.; Pun, S. H.
Biomater. Sci. 2019, 7 (3), 789–797. https://doi.org/10.1039/c8bm01262h
Endosomen in verschiedenen Zelltypen säuern unterschiedlich schnell und in unterschiedlichem Ausmaß an à Konsequenzen für pH-responsive Systeme
Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of MRNA
Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J. P.; Hou, S.; Esposito, A. A.; Ketova, T.; Welsher, K.; Joyal, J. L.; Almarsson, Ö.; Sahay, G.
Nat. Commun. 2020, 11 (1), 1–13. https://doi.org/10.1038/s41467-020-14527-2
Sehr interessantes Paper, in dem viele verschiedene LNP-Komponenten (insbesondere Cholesterol-Analoga) systematisch durchgetestet wurden und versucht wurde eine Struktur-Wirkungsbeziehung zur Transfektion herzustellen.
IL-33/Regulatory T Cell Axis Triggers the Development of a Tumor-Promoting Immune Environment in Chronic Inflammation
Ameri, A. H.; Tuchayi, S. M.; Zaalberg, A.; Park, J. H.; Ngo, K. H.; Li, T.; Lopez, E.; Colonna, M.; Lee, R. T.; Mino-Kenudson, M.; Demehri, S.
Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (7), 2646–2651. https://doi.org/10.1073/pnas.1815016116